
http://www.youtube.com/watch?v=pvFRLIjOLOU
CLICK ON IMAGE ABOVE TO WATCH NEWS DOCUMENTARY
ASPARTAME DANGERS
Interviews with women who became ill ingesting products with aspartame.
|
Food For Thought "Truth Is What Matters" © copyrighted Aspartame Sweetness Disguised as Disease or Death? Also Known As Equal®, Nutrasweet®, or Spoonful® September 7th, 1996 IMPORTANT UPDATES June 25, 2007 October 22, 2007 February 29, 2008 July 20, 2009 - Dec. 15, 2009 - April 25, 2010 - May 1st, 2010 May 30, 2012 | by columnist David Lawrence Dewey "Reading provides knowledge... knowledge leads to answers." Updated July 4, 2004 January 22, 2005 Updated July 14, 2005 Updated October 3, 2005 December 12, 2005 April 9, and 29th 2006 IMPORTANT UPDATE June 25,- Oct. 22, 2007 February 29, 2008 April 11, 2009 May 5, 2010 January 7, 2012 May 07, 2012 |
SEARCH HOME Previous Columns |

Hawaii Senate Chairs and Vice Chairs Co-Sponsor Resolution Asking FDA Commissioner to Rescind Approval for Aspartame Certain members of the Hawaii Senate passed a Resolution authored by Sen. Suzanne Chun Oakland, which requests the Department of Health and National Academy of Sciences to review existing reports and studies related to aspartame, by funding source.
The Resolution states there is an enormous amount of evidence that has been compiled concerning the neurodegenerative harm aspartame can cause, and thus the Resolution request that the U.S. Food and Drug Administration rescinds approval of aspartame immediately on a phase-out basis over six months to one year.
The Resolution, HCR132, introduced by Rep. Josh Green, M.D., Chairman of the House Committee on
Health, was approved first by the Health Committee. The Resolution will then move on to the next government agency, (The Consumer Protection and Commerce). This would set up a work group to explore the need to ban or improve labeling containing aspartame. It was again opposed by Dr. Chiyome Fukino, M.D., Director of the Health Department, an appointee of Republican Governor Linda Lingle, who opposed the House Bill to ban aspartame on the flawed basis of an Ajinomoto-funded review study; Ajinomoto is the world's largest manufacturer of Aspartame, and another proven neurotoxic food additive, Monosodium Glutamate.
Text of Senate Resolution
Requesting the Department of Health and the National Academy of Sciences to review existing reports and studies related to Aspartame, and Requesting the United States Food and Drug Administration to Rescind Approval for United States Markets, carried by Hawaii Senator Suzanne Chun Oakland.
House Health Passes Resolution Creating Aspartame Task Force
UPDATE June 25, 2007
CSPI Urges Consumers To Avoid Aspartame
The CSPI, the Consumer Science Protection Institute issued a warning to consumers today in a press release.
As a result of the new study Ramazzini Foundation, the CSPI downgraded aspartame with their warning to consumers from "use caution" rating to "everyone should avoid aspartame." The CSPI also urges everyone to avoid the artificial sweeteners acesulfame potassium an saccharin. It rates sucralose, also known by the brand name Splenda, as safe, which I do not agree with. Larter on, I'll tell you why Splenda is not safe as well...why? It is basically made from chlorine.
The Ramazzini Foundation new study published in the journal Environmental Health Perspectives, found statistically that aspartame significantly increases lymphomas and leukemias in rats. The rats were fed as little as 20 milligrams of the sweetener per kilogram of body weight which is an amount of what some people consume. The new study is superior to a similar one released in 2005 which exposed rats to aspartame before their birth.
This is a significant move by the CSPI who finally is joining those of us who have informing consumers for over the last ten years not to consume aspartame.
Before we get to the aspartame column below I originally wrote in September, 1996 and which has been continously updated since with new findings...there are important updates and information to the column since 1996 that you need to be aware of. Especially a new study published January, 2007 that shows aspartame alters genes in animals. In addition, listed below is the second Ramazzini Institute Study which confirms AGAIN that aspartame is carcinogenic that was published in January as well. This is the Institute's 2nd study that confirms this.
You will also read about those of us that have been writing about the deadly effects of aspartame since 1986.
Mary Nash Stoddard who started the Aspartame Safety Network in 1986. Stoddard did several CBS 60 Minutes Specials about the dangers of aspartame. Stoddard also wrote "Deadly Deception - Story of Aspartame", a toxicology sourcebook. The book documents the harmful effects of the world's most toxic artificial sweetener. The companion one hour "Deadly Deception" video is further documentation - taped at a prestigious scientific conference. You can purchase both at Stoddard's website. There is Dr. Janet Hull, who has written the book on aspartame called Sweet Poison, and also a Detox book on how to get this deadly chemical out of your body.
It is my opinion that aspartame is as dangerous as hydrogenated oils causing a whole host of diseases including,
coronary heart disease, to diabetes type II, to cancer, autism, food allergies and autoimmune diseases.
I would also like to mention cook book author and playwright, Carol Guiford who has been researching and writing about the deadly effects of aspartame for years. Also, Shoshanna Allison who has made many contributions in research about aspartame for many years. Last, there was Bryant Holman who used to maintain the Aspartame Support Group Message Board. Bryant collected a library of other information about aspartame through the years. Bryant passed away in 2009. UPDATE 4-29-07
DOES A NEW STUDY SHOW THAT ALTERATIONS ON GENES The following study below was published January, 2007 in the Hungary VIVO Journal. The study was performed by the Institute of Public Health in Hungary and can be found on the National Institutes of Health website. It is a very significant study that shows that aspartame ALTERS genes in various organs, especially, lymphoreticular organs, bone-marrow and kidney organs in rats receiving aspartame. Lymphoreticular organs are your immune system, lymph nodes etc. This shows that aspartame ALTERS the genes in these organs, thus, this can result in disease. Any time a gene is altered by any source, chemical, radiation or food allergies, it can cause diseases, including those I mentioned above, including food allergies. Based on this research, I now believe this gene damage can also occur in the womb as the child is developing if the Mother when pregnant is ingesting these chemical food additives and or hydrogenated oils, and this is what is causing the massive increase in autism and food allergies in adults and children over the last ten years.
In Vivo. 2007 Jan-Feb;21(1):89-92.
If the above does not work, here is a .pdf of the study from the NIH website.
The Effect of Aspartame Administration On Gombos K, Varjas T, Orsos Z, Polyak E, Peredi J, Varga Z, Nowrasteh G,Tettinger A, Mucsi G, Ember I.
BACKGROUND:
Aspartame (L-phenylalanineN-L-alpha-aspartyl-1-methyl ester) is an artificial sweetener with wide spread applications. Previously published results have shown that among rats receiving aspartame a significant increase of lymphoreticular neoplasms, brain tumours and transitional cell tumors occurred. The aim of our short-term experiment was to investigate the biological effect of aspartame consumption by determining the expressions of key oncogenes and a tumour suppressor gene.
MATERIALS AND METHODS: After one week per of administration of various doses of aspartame to CBA/CA female mice, p53, c-myc, Ha-ras gene expression alterations were determined in individual organs.
RESULTS: The results showed an increase in gene expressions concerning all the investigated genes especially in organs with a high proliferation rate: lymphoreticular organs, bone-marrow and kidney.
CONCLUSION: Aspartame has a biological effect even at the recommended daily maximum dose.
UPDATE April 17, 2007 Britain's third largest supermarket, Sainsbury announced April 17, 2007 that they will be removing artificial flavours and colours, especially aspartame sweetener, from its private label soft drinks.
IN VARIOUS ORGANS IN ANIMALS OCCUR WHEN
ANY AMOUNT OF ASPARTAME INGESTED?
YES !
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=17354619&query_hl=2&itool=pubmed_DocSum
Oncogene and Suppressor Gene Expressions
Faculty of Medicine, Institute of Public Health University of Pecs, Pecs, Hungary
Email: katalin_gombos@yahoo.com
Britains 3rd Largest Supermarket Removes Aspartame
http://www.beveragedaily.com/news/ng.asp?n=75998-sainsbury-s-soft-drinks-natural
UPDATE - April 17, 2007
Second New Study by The Ramazzini Institute
Confirms Aspartame Is Carcinogenic
Full 2007 Ramazzini Study Details
Dr. Morando Soffritti of the European Ramazzini Foundation presented the results of a new study confirming the carcinogenicity of Aspartame on April 23, 2007 at the Mount Sinai Medical School of New York, where he also received the prestigious Irving J. Selikoff Award.
This new, second, long-term study on Aspartame has been once again, confirms the carcinogenicity of aspartame. See the first study below by the Institute published in July 2005 that showed the carcinogenicity the first time. Yet, the FDA recently issued a press release saying they had reviewed the studies and the FDA still says aspartame is safe to use. How many studies does the FDA need to pull this deadly toxic chemical sweetener off the market?
Aspartame, the artificial sweetener made by Searle/Monsanto was found to cause cancer in laboratory animals already in the original studies that were submitted to the FDA when approval was asked to put it on the market. The justified doubts of the FDA's scientists were overridden when Donald Rumsfeld called in his political markers because he was the CEO of Searle who began manufacturing aspartame. How much money changed hands to get aspartame rushed through and approved at the FDA in 1986?
Another study has been conducted in Spain by Trocho, came to similar results, identifying a transformation of parts of the molecule into formaldehyde as a probable cause. The aspartame industry, through the European Food Safety Authority, has succeeded in diverting attention from these damaging findings, calling them an artifact of the study's design.
Aspartame is an artificial sweetener consumed by hundreds of millions of people worldwide. It is used in over 6,000 diet products including soft drinks, chewing gum, candy, desserts, yogurt as well as in pharmaceuticals, in particular, syrups and antibiotics for children. In 2005, the European Ramazzini Foundation published important experimental data demonstrating the carcinogenicity of aspartame. These data demonstrated for the first time that aspartame is a carcinogenic agent, inducing various types of malignant tumors in rats, even at dose levels currently considered acceptable for humans.
Philippine Congress Tried To Forbid Import and Use of Aspartame Spanish Institute of External Commerce (ICEX).
Paseo de la Castellana 14-16, 28046 MADRID.
April 5th, 2007
RELEASED THRU:
902 349 000
A law promulgated by the Philippine Congress to ban aspartame was narrowly defeated. The law would have forbidden the importing and use, in the country, of aspartame, a sweetener that is between 180 to 200 times more potent than sugar.
It would have also banned distribution of other artificial sweeteners like saccharine. Aspartame is known under the brand names as: Equal, NutraSweet, Equal-Measure, Spoonful. [*] According to the said Law aspartame gives rise to a total of 75% of the negative effects reflected in consumers and other users according to the north American administration of food and alimentation, among others, brain tumours, multiple sclerosis, epilepsy, Chronic Fatigue Syndrome,Parkinsons, Alzheimers and diabetes among others. The ban would have affected all use of this product in any type of consumable and infringement would have carried penalties that go from 9,000 euros to 90,000 euros. Lobbyists for the aspartame industry as they have done in the United States and other countries have used the aspartame industry monetary power
and influence with elected officials to prevent such laws in becoming law in other countries as well.
ENGLISH TRANSLATION IS ABOVE AT:
http://www.icex.es/icex/cda/controller/page/0,2956,35582_13637_16030_298341,00.html
THIS UPDATE IS FROM 4-9-2006
The recent media blitz by the artificial sweetener industry denying aspartame (Equal, NutraSweet) is linked to cancer is simply an attempt to discredit the Italian study released last year that shows aspartame causes leukemia and lymphoma cancers. Researcher, Unhee Lin of the National Cancer Institute says: "the recent NIH-AARP questionnaire was an "unscientific survey."
Read the complete Press Release, HERE - rebutting this NIH-AARP Study and Associated Press reporter, Marilyn Marchione.
PDF copy of the Press Release
UPDATE 7-14-2005
BREAKING NEWS - A DL DEWEY EXCLUSIVE
New - Just Published - Long Term Study Shows Aspartame
Induces An Increase of Lymphomas and Leukemias
A long-term study to evaluate the potential carcinogenic effects of aspartame, an artificial sweetener used in more than 6,000 food and pharmaceutical products has recently been completed in the experimental laboratories of its Cancer Research Center of the European Foundation of Oncology and Environmental Sciences B. Ramazzini in Bologna, Italy. The first results of the experiment were reported to the Ministry of Health and to the Superior Institute of Health of the Italian government in April 2005.
In mid-June, these findings were then communicated to the European Food Safety Authority, the Herbert Irving Comprehensive Cancer Center of Columbia University, the National Cancer Institute of the US government, and the National Toxicology Program of the US National Institutes of Health.
The preliminary results of the study "demonstrate that aspartame, when administered to rats for the entire life span, induces an increase of lymphomas and leukemias in female rats. The study is currently being published in the European Journal of Oncology and final results will be presented at the 3rd international scientific conference of the Collegium Ramazzini, Framing the Future in Light of the Past: Living in a Chemical World”, to be held in Bologna, Italy from September 18-21, 2005, the proceedings of which will be published in the Annals of the New York Academy of Sciences.
You can download the study in .pdf format here:
http://www.ramazzini.it/fondazione/docs/AspartameGEO2005.pdf
Update, July 24, 2004
Was this woman wrongly convicted of murdering her husband, when it fact it was aspartame poisoning?
Read the details:
THE CHARLES FLEMING MURDER CASE
How Did Diane Fleming Get Wrongly
Convicted Of Murdering Her Husband?
 Dr. Janet Starr Hull, PhD
Dr. Janet Starr Hull, PhD
UPDATE 10-1-2005
NEW BOOK JUST PUBLISHED ON SPLENDA®
September 2, 2005
Splenda®: Is It Safe or Not?
Sucralose also known as Splenda® is synthesized by this five-step process:
So sucralose becomes a low-calorie sugar with a complicated process that results in Splenda’s® chemical formula:
1,6-dichloro-1,6-dideoxy-BETA-D-fructofuranosyl-4-chloro-4-deoxy-alpha-D-galactopyranoside.
This is Splenda®. Are you sure now you want to be consuming these deadly chemicals?
Sucalrose is also in many food products, including over the counter drug medications, like Rolaids antacid. Also, Listerine's new Citrus mouth wash. Make sure you read the labels.
In addition, it contains these deadly chemicals, some known to cause cancer in animals, some are even listed on the EPA as poisons:
Acetone - Acetic acid - Acetyl alcohol - Acetic anhydride - Ammonium chloride
Benzene - Chlorinated sulfates - Ethyl alcohol - Isobutyl ketones
Formaldehyde
Hydrogen chloride - Lithium chloride
Methanol - Sodium methoxide
Sulfuryl chloride - Trityl chloride - Thionyl chloride - Toulene
You can read the rest of details and research findings explained in laymens terms in:
Dr. Hull's new book - Splenda®: Is It Safe Or Not? can be purchased through Amazon.com:
~ Click Here To Order ~
This is a must read -
Mothers - make sure you read this for your children's health!
Dr. Hull's new book is getting rave reviews for exposing this deadly chemical!
I give it a TEN ! Outstanding - well written and researched !
Visit Dr. Hull's website, Is Splenda Safe Or Not?
More on Dr. Janet Hull PhD
 Dr. Janet Hull PhD
Dr. Janet Hull PhD
http://www.janethull.com - is an aspartame victim herself. Her aspartame expertise is based on her professional background. She holds a Doctorate in Nutrition, a Master's Degree in Environmental Science, is an international geographer and geologist, a former university professor, firefighter and Hazardous Waste Specialist and Emergency Responder. She is a Licensed Certified Nutritionist, certified fitness professional and author.
Dr. Hull's diverse background supports her unique approach to contemporary nutritional issues. She has dedicated the past ten years to sharing with others her life-threatening experience and natural recovery from aspartame poisoning.
 Sweet Poison, written by author Dr. Janet Starr Hull, is a book exposing aspartame dangers. SweetPoison.com provides a variety of aspartame information including nutritional advice on aspartame detoxification, aspartame side effects and up-to-date information on aspartame dangers.
Sweet Poison, written by author Dr. Janet Starr Hull, is a book exposing aspartame dangers. SweetPoison.com provides a variety of aspartame information including nutritional advice on aspartame detoxification, aspartame side effects and up-to-date information on aspartame dangers.
Order the books through Barnes and Noble or Amazon.Com
For more information, go to: http://www.sweetpoison.com
 Dr. Hull has also written the book:10 Steps To Detoxification
Dr. Hull has also written the book:10 Steps To Detoxification
http://www.detoxprogram.net
Dr. Hull has combined five primary nutritional components that remove toxins, replace nutrients depleted by toxicity, and restore a natural state of health. It is like peeling the layers off an onion, as each layer is removed, the underlying layers reveal what’s really behind disease symptoms. The deeper you go toward the ‘core’ of the problem, healing becomes long-term reality.
How Can the Detox Program Help You?
The Detox Program identifies the toxin or toxins present within the body and removes the toxins via the water stores, urine, fecal wastes, and the bloodstream. Most toxins can be removed if the program is followed for the duration the toxinsare present within the body tissues, and The Detox Program is so natural, it can be used for your lifetime as a maintenance program for future chemical exposures.
 Dr. Janet Starr Hull also presents The Richardson Cancer Diet, an alternative cancer diet to help the body fight cancer. The book features cancer fighting foods, tools, nutritional recommendations, resources and more. Learn more at:
Dr. Janet Starr Hull also presents The Richardson Cancer Diet, an alternative cancer diet to help the body fight cancer. The book features cancer fighting foods, tools, nutritional recommendations, resources and more. Learn more at:
http://www.alternativecancerdiet.com
Make sure you visit Dr. Hull's sites for further information:
http://www.hullisticnetwork.com
http://www.hullisticweb.com
Read about Dr. Hull's hair analysis tests:
http://www.hairanalysisprogram.com
Subscribe to Dr. Hull's Healthy Newsletter:
Here you will find a treasure trove of information
about aspartame, studies, chewing gums etc.
http://www.healthynewsletter.com


Mary Stoddard and Washington D.C. Attorney, Jim Turner's
Aspartame Consumer Safety Network .
Mary was the first consumer advocate in 1987 before all others
in educating consumers about this deadly chemical sweetener.
Stoddard's and Turner's site provide eye opening information about the artificial sweetener, aspartame - presented in a rational, intelligent manner. Both are the original pioneers of the international anti-aspartame movement. Journalist, Mary Nash Stoddard and Washington attorney, James Turner, Esq. have been instrumental in educating the general public around the world as to the reported dangers of aspartame. Their website is where people go to when they have questions about this issue and need answers from the experts.
 About Mary Nash Stoddard
About Mary Nash Stoddard
Author/Broadcast Journalist/Expert Medical Witness/Food Safety Consultant
Mary co-founded the massive international anti-aspartame movement in the mid 1980's, following the brain tumor death of her forty two year old husband, Mike.
In 1985, Ms. Stoddard suffered a life threatening aspartame-related blood disorder, whereupon, The NutraSweet Co. offered her an all-expense paid vacation for two anywhere in the world, if she would agree to be tested by their doctors. She declined, with the blessing of her doctor, and the rest is history. She has conducted multi-national lecture tours and is a popular visiting professor at colleges, universities and medical schools.
Stoddard's efforts over the past nearly two decades has led to the present rejection of the sweetener by many of the food and beverage giants of industry. These companies rush to distance themselves from the liabilities associated with use of a neuro-toxic substance in their products. She has testified in court as an Expert Medical Witness and like her famous counterpart, Erin Brockovitch, has helped with a number of lawsuits on behalf of consumers. Her powerful message has reached millions around the world through the airwaves on radio and television, in print and personal appearances.
Stoddard also wrote "Deadly Deception - Story of Aspartame", a toxicology sourcebook, edited by Ms. Stoddard. The book was the first book written about the dangers of aspartame. The book documents the harmful effects of the world's most toxic artificial sweetener. The 250 page toxicology sourcebook is endorsed by doctors, scientists, medical reporters and mentioned by Dr. Robert Atkins on his radio show in New York. The companion one hour "Deadly Deception" video is further documentation - taped at a prestigious scientific conference. You can purchase both, as well as audio tapes and CD's at Stoddard's website.
Make sure you read Stoddard's recent article concerning her warnings of the recent decision of the IADSA to increase food additives in foods, even in babies foods.
ARTICLE: Reckless Endangerment of World's Food Supply By IADSA
Make sure you also visit this website, Aspartame Support Group And here is the online Support Aspartame Group Message Board: http://groups.yahoo.com/group/aspartame
Bryant Holman, the webmaster, has collected a library of other articles about aspartame and it also provides an online Support Group to help and answers questions about aspartame.
You can view a trailer about the documentary here: If you have not watched Cori Bracket''s documentary, Sweet Misery about her own experience with aspartame and how she recovered from MS, then I strongly suggest you do. Sweet Misery was released by Cori and her husband in June of 2004. Their primary investigation includes interviews with doctors, lawyers, people who have had health probems which they associate with aspartame usage, advocates, and many others.
The website is: http://www.soundandfury.tv/pages/sweet%20misery.html
If you have not watched Cori Bracket''s documentary, Sweet Misery about her own experience with aspartame and how she recovered from MS, then I strongly suggest you do. Sweet Misery was released by Cori and her husband in June of 2004. Their primary investigation includes interviews with doctors, lawyers, people who have had health probems which they associate with aspartame usage, advocates, and many others.
The website is: http://www.soundandfury.tv/pages/sweet%20misery.html
http://www.sweetremedy.tv/pages/smtrailer.html
FACT: Aspartame is banned in Europe for all children's products.
Why is not in the U.S.?
Parents - read all labels of products your children are consuming or ingesting.
Aspartame
Sweetness Disguised as Disease or Death?
Also Known As Equal®, Nutrasweet® and Spoonful®
by David Lawrence Dewey
© September 7th, 1996
Continously updated through 2008
- All Rights Secured
This column is protected by International Copyright Laws.
The article may not be copied into newsgroups or emails, or copied onto another website.
 USE AT YOUR OWN RISK
USE AT YOUR OWN RISKNow To My Aspartame Column...
Since 1970, more than 100 research studies have confirmed serious adverse reactions to Aspartame. Yet...aspartame was given approval by the FDA for limited use on July 26, 1974 as a free flowing substitute, tablets for sweetening hot beverages, ( this is deadly because heat creates the neuro-toxin methanol ), cereals, gum, and dry bases.
Aspartame was not approved for baking goods, cooking or carbonated beverages at this time. It was finally approved by the FDA for use in dry foods in 1981 and carbonated soft drinks in 1983.
In a 1993 act, the FDA approved aspartame as an ingredient in numerous foods which would always be heated to 86F degrees or above. Prior to this and for over the next eight years, scientists at the FDA refused to approve it because of the seizures and brain tumors this chemical drug produced in lab animals.
The FDA continued to refuse to approve it until President Reagan took office. Reagan fired the FDA Commissioner who wouldn't approve it and appointed Dr. Arthur Hull Hayes as the new FDA Commissioner. Despite strong objections and much opposition, a Public Board of Inquiry was finally set up. The Board issued their findings, "Do not approve aspartame". However, Dr. Hayes over turned his own Board of Inquiry. Aspartame is, by far, the most dangerous substance in the market that is added to foods. Aspartame accounts for over seventy-five percent of the adverse reactions to food additives reported to the FDA.
There have been over 10,000 citizens submitting adverse reaction reports to the FDA since 1982. (DHHS 1993b, DHH@, 1995). These are just a few of the 92 symptoms.
Headaches/Migraines, Seizures, Numbness, Weight Gain, Depression, Irritability, Insomnia, Hearing Loss, Breathing Difficulties, Slurred Speech, Tinnitus, Memory Loss, Dizziness, Nausea, Muscle Spasms and Cramps, Rashes, Fatigue, Tachycardia, ( fast heart beat ), Vision Problems, Heart Palpitations, Anxiety Attacks, Loss of Taste, Vertigo, ( causing airplane pilots to have problems flying ), Joint Pain.
The following is an image of the original FDA summary of complaints from the
Department of Health and Human Services, April 20th, 1995.
This document is a no longer available from the archives registry.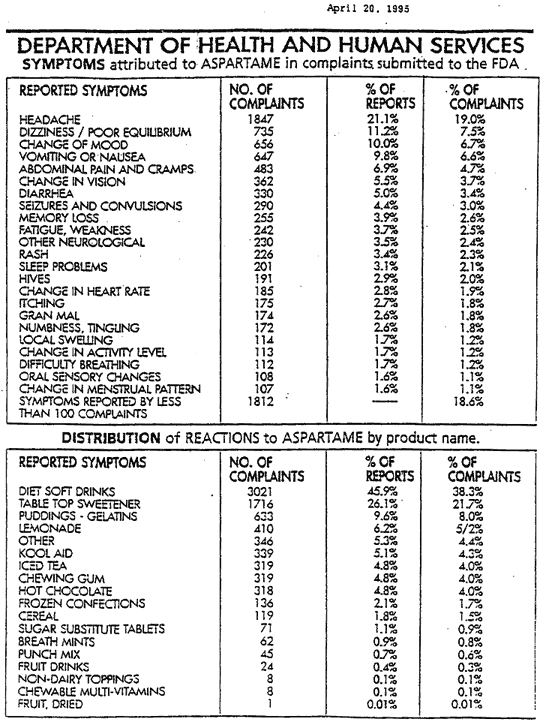
What makes matters worse is the FDA admits that less than 1% of serious drug or food adverse reactions are reported to the FDA, ( Kessler 1993). This represents that if 10,000 reports were filed from 10,000 people, the actual number of people that should have reported reactions would have been more than one million people that did not report reactions. This being the case, then why is Aspartame still allowed to be in foods?
Many of these reactions are very serious. They include gran mal seizures and death as disclosed in a February 1994 Department of Health and Human Services report. This column covers the massive fraud, deception and cover up by our own government agency and politicians.
If you are truly concerned about your health and want to see how corruption at the highest levels of our government played a role in the approval of this deadly chemical for human consumption, then please read on.
Here are the facts.
What Exactly Is Aspartame?
Aspartame, ( C14H1805 : L-Aspartyl-L Phenylalanine Methyl Ester, is its official chemical name ), is made up of three chemicals, 40% - Aspartic Acid, 50% - Phenylalanine and 10% - Methanol. It is argued by those who want you to believe that Aspartame is something good is because they state that phenylalanine and aspartic acid are important amino acids and are commonly found in many foods bound to proteins. However, there are people who are very sensitive to phenylalanine. It causes a disease called PKU which can be fatal.
There is a huge problem with anyone saying that Aspartame is good. These amino acids in Aspartame are absorbed and metabolized differently from those found in normal foods. Why? Proteins in foods are broken down very gradually and amino acids which comprise a full range of proteins are gradually absorbed slowly. This gradual absorption leads to a very slow and small increase in some of the plasma amino acids into cells.
With Aspartame however, the aspartic acid and phenylalanine are free and unbound to any proteins. They are very quickly absorbed into the human body. This causes a rush of amino acids into the system which leads to spikes in plasma amino acids. For reference pertaining to this process, refer to the medical journal Metabolism (36/5):507-512.
Some fruit juices and alcoholic beverages contain small amounts of methanol which is 10% of Aspartame. It is very important to remember, methanol never appears alone. In every case, ethanol is present and usually at much higher amounts. Ethanol is an antidote for methanol toxicity to the body. All natural foods that contain methanol contain ethanol. It is mother natures way of protecting the body from methanol toxicity.
However...in Aspartame, THERE IS NO ETHANOL to counter the methanol that is produced!
Aspartame is made of phenylalanine (50% by weight) and aspartic acid (39%), both ordinary amino acids, bound loosely together by methanol (wood alcohol, 11%). Similar amounts of methanol are found in many fruits and vegetables and is locked up in complex pectin molecules, however, this methanol from natural food sources is always paired with ethanol, the ethanol is the body's natural antidote to the methanol from food sources and are not usually released by human digestion and so the this methanol from food sources is harmless.
This is different though with aspartame. The readily released methanol from aspartame is within hours largely turned by the liver into formaldehyde and then formic acid, both potent, cumulative toxins. In 1998, C. Trocho and his team proved the methanol in aspartame converts to formaldehyde, accumulating in the liver and fatty tissue.
Aspartame converts to formaldehyde in vivo in the bodies of laboratory rats.
This study by C. Trocho et al, is sometimes referred to as the "Barcelona report", or the "Barcelona study". It was conducted by the staff of the Biology Department of the University of Barcelona. This study clearly shows that aspartame which was labeled with a carbon 14 isotope was transformed into formaldehyde in the bodies of the living specimens and that when they were examined later, the radioactive tagged formaldehyde was spread throughout the vital organs of their bodies. This conclusively proves that aspartame does indeed convert to formaldehyde in the bodies of aspartame consumers, and that many of the symptoms reported by victims of aspartame toxicity are indeed those associated with the poisonous and cumulative effects of formaldehyde.
As clearly stated by the title of the report .....
"FORMALDEHYDE DERIVED FROM DIETARY ASPARTAME...
BINDS TO TISSUE COMPONENTS IN VIVO"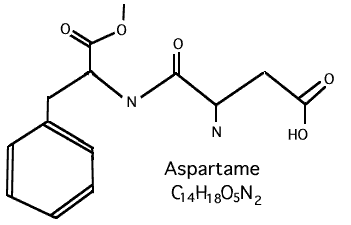
Aspartame is 11% Methanol
This 11% equals out to about 1,120 mg of aspartame in a 2 liter diet soda, or the equivalent to six 12-oz cans of diet soda. This creates a total of 123 mg methanol (wood alcohol) from this 2 liter bottle or six cans. In addition, about 30% of the methanol remains in the body as cumulative durable toxic metabolites of formaldehyde and formic acid. This is 37 mg daily, a gram every month that is accumulating in and affecting every tissue in the body.
If only 10% of this methanol from aspartame accumulates daily as formaldehyde in the body, that would mean a total of 12 mg daily of formaldehyde that is accumulating in the body. This is about 60 times more than the 0.2 mg from 10% retention of the 2 mg EPA daily limit for formaldehyde in drinking water.
And remember, the EPA limit for formaldehyde in drinking water is 1 ppm, or 2 mg daily for a typical daily consumption of 2 Liter of water.
A lab using gas chromotography can easily identify what is called the NTF1 footprint of the aspartame as well as the amounts of methanol, formaldehyde and formic acid in a 2 liter bottle and or 6 cans of diet soda. It can also establish the deadly concentration of aspartame and its by-products of methanol, formaldehyde and formic acid. And keep this in mind. If the bottle or cans of a diet soda have been bouncing around in a truck during the got summer months, the hotter it gets, the more the sift drink will break down into these deadly chemicals inside the bottles or cans.
An 11 Year Old 6th Grader, Jennifer Cohen Proved This Point...
In 1997, Jennifer conducted an experiment proving that aspartame, the artificial sweetener in diet soda, breaks down into two deadly neurotoxins when stored at room temperature and under refrigeration.
These were Jennifer's findings:
Her Method Of Analyzing The Levels Of Chemicals
Jennifer purchased (24) cans of Diet Coke from a local supermarket.
She put 7 cans in a refrigerator;
7 cans in a room at room temperature (about 69 degrees) and;
7 cans in a BOEKEL incubator (80 Watts, 120 AC volts, 0.75 Amps, catalog # 131500). She set the temperature on the BOEKEL incubator at 40 degrees Celsius which is 104 degrees Fahrenheit.
Jennifer left them in all the locations for 10 weeks (70 days). She placed a thermometer next to each group of cans checked and recorded the temperatures daily. She chose that temperature because in 1985, the National Soft Drink Association reported a similar experiment in which diet soda stored at that temperature had indeed turned into formaldehyde.
Jennifer then performed a double blind study. With her mothers help, they took a can from each of the (3) test groups of cans, including a brand new can of Diet Coke. They placed a number on the four cans. She put all of the cans in a cooler and covered them with ice so that all of these previous test group cans would be served at the same temperature. She performed the sample rating experiment with 10 human test subjects again. Jennifer did not know which sample each person was drinking. Jennifer gave each person a small cup sample from each of the (4) cans that simply had a number on them.
These are the results from the second sampling and rating by the 10 human test subjects:
| SUBJECTS | Room Temp Sample #517 |
Cold Temp Sample #520 |
WarmTemp Sample #520 |
New Can Sample #520 |
| SUBJECT # 1 | 4 | 4 | 4 | 3 |
| SUBJECT # 2 | 3 | 2 | 4 | 1 |
| SUBJECT # 3 | 2 | 3 | 3 | 2 |
| SUBJECT # 4 | 1 | 2 | 4 | 2 |
| SUBJECT # 5 | 2 | 2 | 4 | 1 |
| SUBJECT # 6 | 2 | 3 | 4 | 1 |
| SUBJECT # 7 | 2 | 3 | 3 | 4 |
| SUBJECT # 8 | 3 | 2 | 4 | 3 |
| SUBJECT # 9 | 3 | 2 | 4 | 1 |
| SUBJECT # 10 | 3 | 3 | 4 | 2 |
| AVERAGE | 2.5 | 2.6 | 3.8 | 2.0 |
Once again, these were the findings of Winston Laboratories concerning the (3) controlled temperature groups of Diet Coke.
In the samples that had previously been in the refrigerator for (70) days, all that was left of the 0.06 per cent of the aspartame was 0.058 percent. The testing showed that the aspartame had turned into 0.001 percent DKP and 53.5 parts per billion of formaldehyde by sitting in the refrigerator for (70) days.
In the samples that had been in Jennifer's room can for (70) days , all that was left of the 0.06 percent aspartame was 0.051 per cent. The testing showed that the aspartame had turned into 0.002 percent DKP and 231 parts per billion of formaldehyde after sitting in room temperature for (70) days.
In the sample that had been in the incubator for (70) days at 104 degrees, all that was left of the 0.06 percent aspartame was 0.026 percent. The testing showed that the aspartame had turned into 0.010 percent DkP and 76.2 parts per billion in the formaldehyde after sitting in the incubator at 104 degrees for (70) days.
* The sample of the new can of Diet Coke was not re-tested by the lab after this double blind study of a sample from each of the (3) groups of previsouly controlled temperature cans. It was used for the taste test only. The baseline can was not tested for formaldehyde or DKP because it was assumed that FDA would ban any new product containing poison.
Jennifer spent $1250.00 in lab testing to prove this. This may not be a lot of money to a drug company but it sure was to an 11 year old. Jennifer wound up baby-sitting the summer of 1997 to pay for this excellent study that showed the following:
MOST IMPORTANTLY - JENNIFER'S STUDY SHOWED:
Jennifer's study showed also that temperature creates two effects with aspartame.
First, the higher the temperature of storage, the higher the level of DKP in the soda.
Second, cans sitting in room temperature creates the highest levels of formaldehyde in diet soda
Third, at very high temperatures, the formaldehyde breaks down.
Fourth, diet soda even stored in a refrigerator at cold temperature, the aspartame breaks down into formaldehyde.
Jennifer's study shows without doubt, that after diet soda containing aspartame is purchased, it should not be stored in the heat, like in a garage or under any condition for a long period of time. Jennifer's efforts in her study showed without doubt that further research should have performed with more samples at different temperatures for different time periods so that safety levels could be determined.
But the " aspartame industry" has never done this type of testing because as Jennifer's study proves, the results would showed how aspartame breaks down into these deadly other chemicals of methanol, formaldehye and DPK.
Jennifer made a comment after she completed her study.. She said, "The FDA says, concerning aspartame, that they believe that based on all the information that we received to date that this is a safe product."
Jennifer's comment to the public, "Decide for yourself."
The principal of Oradell Public School where Jennifer conducted her study was Scott Ryan.
What Does Aspartame Do In The Body - It Is Another Excitotoxin?
Aspartate acts as a neurotransmitter in the brain by facilitating the transmission of information from neuron to neuron.
Aspartic Acid which is 40% of Aspartame, is an amino acid. When it is taken in its free form as in Aspartame, ( unbound to proteins ), it significantly raises the blood plasma levels of Aspartate. This excess Aspartate leads to a high level of this neuro transmitter in certain areas of the brain. Too much Aspartate in the brain kills certain neurons by allowing the influx of too much calcium into the cells. This influx then triggers excessive amounts of free radicals which literally kills brain cells. The neural cell damage that can be caused by excessive asparate or glutamate, ( glutamate known as MSG, is another excitotoxin ). These exicotoxins excite or stimulate neural cells to literal death! Some areas of the brain affected by these spiked levels of aspartate ARE NOT protected by the blood brain barrier which protects the brain. A large majority, 75% or more of neural cells in particular areas of the brain are killed before any clinical symptoms of a chronic illness are finally seen. Aspartic Acid has a cumulative harmful effect on the endocrine system and reproductive systems as well. Several animal experiments show that exicotoxic amino acids can penetrate the placental barrier and cause damage to the fetus, the unborn child. These same excitotoxins also dramatically lower the male and female hormones testosterone and estrogen. DNA damage to sperm has been reported from Aspartame use.
In both human and animal study experiments, plasma asparate levels are shown to spike to high levels after liquid administration of Aspartame. Humans are five times more susceptible to aspartic acid and glutamic acid, (MSG) than rodents, and twenty times more susceptible than monkeys. Why? Because humans concentrate these excitatory amino acids in their blood plasma at much higher levels and for a longer period of time.
These are just a few of the many chronic illnesses caused by long-term exposure to these excitotoxin amino acids that are found in Aspartame. Remember that asparate acid is 40% of Aspartame.
Multiple Sclerosis, Hormonal Problems, Hearing Loss, Epilepsy, Alzheimer's Disease, Amyotrophic Lateral Sclerosis, (ALS - Lou Gehriig's Disease), Parkinson's Disease, Hypoglycemia, AIDS Dementia, Brain Lesions and Tumors, Neuroendocrine Disorders. I wonder, could all that cereral Lou Dehrig ate which contain glutamic acid which was added as a preservative caused his disease?
Phenylalanine - 50% Of Aspartame
Phenylalanie is an amino acid normally found in the brain. Persons with the genetic disorder phenyletonuria, (PKU), cannot metabolize phenylalanine. This leads to dangerously high levels of pheylalanine in the brain which can be lethal. It has been shown that ingesting Aspartame, especially with carbohydrates, can lead to excess levels of phenylalanine in the brain. Even in persons that do not have PKU, human testing has shown that phenylalanine levels of the blood were increased significantly in humans test subjects who used Aspartame regularly. Even a single dose of Aspartame raises blood phenylalanine levels. This study, "Dietary Phenylalanine and Brain Function", was performed by Dr. Wurtman and Dr. Walker. They presented their findings at the 1st International Meeting on Dietary Phenylalanine and Brain Function in Washington D.C., May 8-10, 1987. Excessive levels of phenylalanine in the brain also cause the level of serotonin to decrease. This can lead to emotional disorders such as depression. Could this be why today, the largest number of people in this country are being treated for depression? In addition, the decrease in serotonin results in carbohydrate cravings which can lead to increased consumption of carbohydrates, i.e. weight gain. Normal levels of serotonin blunts the sensation of craving carbohydrates. With serotonin decreased, weight gain and excess weight that never goes away regardless of how one diets is seen. Serotonin is part of the feedback system that controls metabolism. In his testimony before the U.S. Congress, Dr. Louis J. Elsas showed that high blood phenylalanine can be concentrated in parts of the brain. This can be very dangerous for infants and fetuses. He also showed that phenylalanine is metabolized much more efficiently by rodents than humans, thus making it even more dangerous for humans. The hypothalmus, the medulla obolngata and corpus straitum areas of the brain received the largest concentrations of phenylalanine buildup. This excessive buildup can cause schizophrenia and make one more susceptible to seizures and even brain tumors.
Aspartame Causes Brain Tumors
In the early 1970's, Dr. John Olney testified before Congress that Aspartame causes brain tumors.
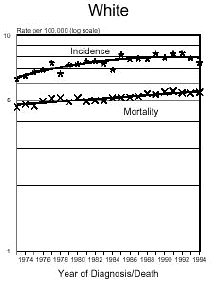
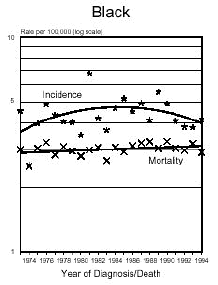
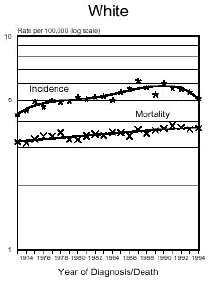
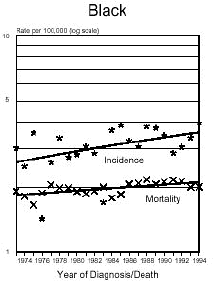
Below is a graph provided by the National Cancer Institute (SEER) On December 5, 1975, the FDA put a hold on the approval of Aspartame due to the preliminary findings of the FDA Task Force. During this time period of July 26, 1974 to December 5, 1975, Searle had over $9 million dollars of inventory on hand to dispose of. Where did it go? Searle claims nothing entered the market place during this time even though there have been these reports.
A man in Augusta, Ga. who helped build the NutraSweet plant there says Searle was indeed selling it. There are also reports of ads running for gumballs with aspartame in them.
In addition and what is interesting is that NASH, a non-alcoholic liver disease, was not discovered until 1980. Evidently, Searle was selling aspartame and it was being put into foods and this create the disease the medical community named, NASH. Bottom line...non-alcoholic liver disease is caused from the wood alcohol in Aspartame. Did Searle sell the $9 million dollars of inventory or throw it away? I don't think so! Why?The graph below shows how brain tumors increased significantly during this time frame. What do you think happened?
Aspartame was not approved for baking goods, cooking or carbonated beverages at this time. It was approved for use in dry foods in 1981 and then for carbonated soft drinks in 1983. Incidence means diagnosis, mortality means deaths. What is seen is an increase of brain turmors starting in 1975. This is the year that (MSG), glutamic acid was used more and more in foods. MSG is another deadly excitotoxin. After 1984, a substantial increase in brain tumors can be seen as well. According to published statistics in the Vital Health Statistics, Centers for Disease and Control, National Center for Health Statistics, - Series 3, #30, April 1995, These are the findings:
In 1970... In 1985... Diseases of the retina of the eye in 1970 were 833 cases per 100,000 population. In 1988 it was 1,229 per 100,000. This is a 150% increase in five years after the introduction of aspartame in soft drinks. Both diseases have continued to increase since 1985. Dr. H.J. Roberts published an abstract in 1995 entitled, "Aspartame and Hyperthyroidism A Presidential Affliction Reconsidered ". It stated, "The consumption of products containing aspartame, ( NutraSweet ), by health conscious persons, coupled with a marked decrease in caloric intake and physical activity, may trigger primary hyperthyroidism, ( Graves Eyes Disease )." This statement was based on two biologically unrelated stepsisters along with another patient of Dr. Roberts.
Because of what Dr. Roberts was seeing in his patients, Roberts began warning patients with this disease that they should stop using aspartame that was added to foods and be observed for a possible remission before radioiodine treatment to destroy their thyroid or surgery to remove their thyroid.
Dr. Roberts also reported in an abstract that Carpal Tunnel Syndrome is caused due to Aspartame induced disease. Interestingly enough, Japan has seen much higher increases in both diseases since 1984.
When Diet Coke and Diet Pepsi were introduced in Japan in 1984, it became the drink of the younger generation in Japan. Disease rates caused from asparatme poisoning have skyrockted since then in Japan.
The following is information which validates the increase in brain tumors. White men and women in the United State have been one of the largest consumers of regular sugar sweetened soft drinks. However, black women were the first largest group of consumers of diet soft drinks sweetened with Aspartame.
In the United States beginning in 1986, there was a decline in the black men population as well as white women rates of brain tumors. However, the black woman rate continued to soar. Shortly afterwards, brains tumors in white men and women began to climb as well. If you study how different segments of the population, white, minority, overweight people and their eating habits, much of this can be explained. The decrease in white woman having brain tumors did not decline until late 1991 and 1992, after much had be written about the dangers of Aspartame in the late 80's. The white women population have always responded faster to issues such as foods or things that are not healthy than minority populations.
( http://seer.cancer.gov/Publications/CSR7394/brain.pdf ),
for men and women reflecting the increases of brain tumors in this country from 1974 to 1994. Remember, Aspartame was approved only for limited use in 1974 as a free-flowing substitute, tablets for sweetening hot beverages,cereals, gum, and dry bases. It is important to remember though that heat creates methanol.
( http://www.cdc.gov/nchs/data/series/sr_03/sr3_30.pdf, ) there is a huge increase in cerebrovascular diseases.
372 cases per 100,000 population of cerebrovascular diseases were reported from hospitals.
686 cases per 100,000 population. This is a 185% increase.
(DKP) - A Breakdown Of Phenylalanine
A Dangerous Brain Tumor Causing Chemical
DKP, also known by its chemical name, Aspertylphenylalanine Diketopiperaine is a breakdown product of phenylalanine.
Dr. John Olney saw that DKP when nitrosated in the gut, produced a compound which is similar to N-nitrosourea. This is a powerful brain tumor causing chemical. In addition, DKP has been implicated as a cause of uterine polyps and changes in blood cholesterol by FDA Toxicologist, Dr. Jacqueline Verrett. Remember her name for later, she testified before the U.S. Congress against Aspartame. However, her testimony was ignored.
Two pre-approval studies of Aspartame showed an unusual large number of brain tumors in test animals. Those studies were named, E33/34 and E70. Twelve brain tumors were found in the experimental animals. Dr. Olney, a board certified neuropathologist, testified, "I know that spontaneous brain tumors in laboratory rats are extremely rare, so this is alarming." The archival literature documents an incidence diagnosis not exceeding 0.6%. Since the above incidence rate in NutraSweet fed rats is 3.75%, this strongly suggests that NutraSweet, [aspartame], may cause brain tumors. It certainly suggests the need for additional in-depth research to rule out that possibility in humans. Dr. Olney's testimony and warnings were also ignored.
In 1991, Dr. H.J. Roberts published an article in the Journal of Advancement in Medicine.His findings showed a correlation between the suddenly rising incidence of Primary Brain Cancer and Primary Brain Lymphoma soon after Aspartame was put on the market. In addition, severe adverse effects of Aspartame use by Parkinson's Disease patients has been reported to the FDA and to several independent organizations. Excitotoxins have been implicated in the development and worsening of Parkinson's Disease, ( Blaylock 1994, Choi, 1992, Kurland, 1988 ). It is sad that actor Michael J. Fox who pitched Diet Pepsi in commercials and drank it for years is suffering from Parkinson's Disease. I wonder if he knows that it could very will be the Asparatme that has caused his disease? A personal message to Michael. I suggest you explore this possibility.
Methanol ( aka wood alcohol/poison - 10% in Aspartame )
Methanol is gradually released in the small intestine when the methyl group of Aspartame encounters the enzyme chymotrypsin. The absorption of methanol into the body is sped up considerably when free methanol is ingested. Free, meaning Aspartame. Free methanol is created from Aspartame when it is heated to 86F degrees or higher. This occurs when foods containing Aspartame, including most gum products as well as soft drinks are stored improperly or when heated. What is interesting is this. All those diet soft drinks that are sweetened with aspartame, ride around in delivery trucks in 100 degree weather or stored in hot warehouses. Now do you see the problem?
When aspartame is absorbed quickly as free methanol in the small intestine, the total amount of methanol absorbed will be approximately 10% of the Aspartame ingested.
An EPA assessment of methanol states, " methanol is considered a cumulative poison due to the low rate of excretion once it is absorbed." The absorbed methanol is then slowly converted to formaldehyde by alcohol dehygrogenase in the liver. Yes, you read that right...I said formaldehyde. This formaldehyde is then converted to formic acid by aldehy dehydrogenase in the liver by formaldehyde dehydrogenase in the blood, or through the tetrahydrofolic acid dependent one carbon pool. Methanol thus breaks down into two things in the body. Formic acid, and formaldehyde which is embalming fluid. Basically, your embalming yourself even before your dead if your drink or eat anthing that has Aspartame in it.
Formaldehyde Is A Deadly Neurotoxin!
Formaldehyde a known carcinogen. It is known to cause Squamous Cell Carcinoma in experimental animals. It causes retinal damages, interferes with DNA replication and causes birth defects. Formaldehyde stores itself in fat cells, particularly on the hips and thighs. It is potentially toxic to the retina and optic nerve. These organs are highly vulnerable to metabolic disturbances and neurotoxins because of their unique metabolic requirements. Methanol and its by products cause swelling of the optic nerve and degeneration of ganglion cells in the retina. Repeated exposure to low doses of formaldehyde are shown to cause a wide range of health problems, ( John 19194, Liu 1991, Molhave, 1986, National Research Council 1981, page 175-220, Sirvasta 1992.
Researchers, John, Liu and Molhave stated in their study, "Complaints pertaining to gastrointestinal muscle skeletal and cardiovascular systems were also more frequent in exposed subjects. In spite of formaldehyde concentrations being well within prescribed ACGIH, ( American Conference of Governmental Industrial Hygienists ) limits of 1ppm, the high rates of sickness emphasize the need for detailed studies on formaldehyde exposed subjects."
Formaldehyde appears to be much more toxic to the body in small amounts than formic acid. This is derived from aspartic acid, (Aspartame). The National Council Research Council, 1982, page 179, stated the following about formaldehyde:
"Some adverse effects of formaldehyde may be related to its' high reactivity with amines and formation of methanol adducts with nuclei acids, histones, proteins, and amino acids. The methyanol adducts can react further to form methlene linkages among these reactants. It appears that before formaldehyde reacts with amino groups in RNA, the hydrogen bonds forming the coiled RNA are broken. Formaldehyde reacts with DNA less frequently with RNA because the hydrogen bonds holding DNA in its' double helix are more stable. Reaction of formaldehyde with DNA has been observed by spectrophotometry and electron microcopy. This results in irreversible denaturation. In reactions with transfer RNA, formaldehyde interferes with amino acid acceptance. The equilibrium reaction of formaldehyde with DNA involves thermally activated opening and closing of hydrogen bonds between matching base pairs in the helix. If permanent cross links are formed between DNA reactive sites and formaldehyde, these links interfere with the replication of DNA and may result in mutations."
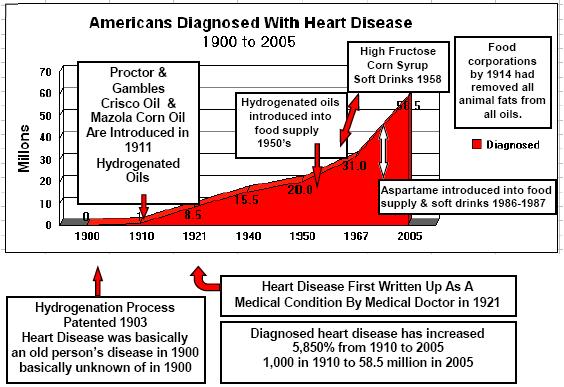
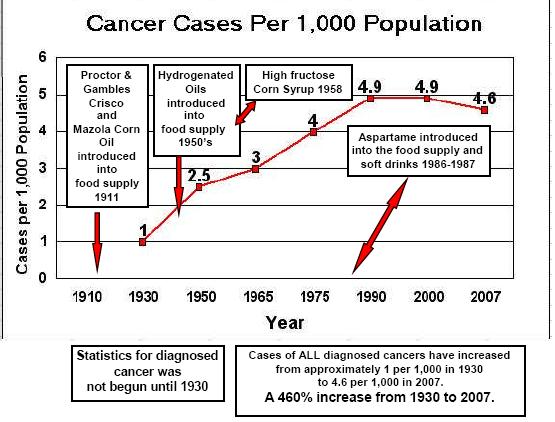
If you have not read by column, "Hydrogenated Oils-Silent Killers", you will understand how the process of hydrogenating oils changes the molecular structure of natural essential fatty acids. Hydrogenation changes the molecular structure of the oil from a positive and negative atom to two negative atoms. This creates an incorrect matched base pair in the helix of the essential fatty acids. These are then formed into malformed amino acids. All of this ties together. If you used Aspartame, and your diet consists of many foods containing hydrogenated oils, you are literally adding a "furnace" creating many immune system problems. Not to mention a substantial increase in coronary heart disease and also setting the stage for diabetes type II and auto-immune disorders.
It is now thought by many researchers that persons with certain illnesses are suffering from what is called formaldehyde toxicity. This is when excess methalanine and semicarbazide synthesize amine oxidase (SSAO) reacts to form formaldehyde. Their findings suggest that formaldehyde, the deaminated product of methlamine may be very well be responsible for these toxic effects. Both SSAO activity and methlamine levels have been reported to be increased in the blood of diabetic individuals. It appears an abormal metabolism of methylamine may be involved in endotheial injury and will subsequently induce athersclerotic plaque formation in the heart, thus causing cardiovascular disorders.
Therefore, regular ingestion of aspartame is most likely adding formaldehyde fuel to the fire. Combine this with foods containing hydrogenated oils and you have not just a furnace, but a roaring forest fire in the human immune system.
We Know What Formaldehyde Does To The Immune System
Dr. Fukimaki and Voldani in 1922 showed immune system alteration from exposure to formaldehyde. In addition, Dr. Sherry Rogers in 1990 went on to prove how urea foam formaldehyde insulation can cause significant damage to the immune system. She found adverse health effects to formaldehyde exposure at levels between .012-1/6 ppm.
Therefore, the formaldehyde metabolite of the methanol in Aspartame is what is causing the slow and silent damage, especially in combination with the other breakdown products of Aspartame. Again, add hydrogenated oils to this deadly neuro-toxin and you have a forest fire of illnesses raging in the human body. If this is the case, formic acid measurements most likely will not tell us what we need to know about the damage being done by the formaldehyde buildup. We need to look also at the Formic Acid buildup in the body.
Formic Acid Causes Cell Death
Exposure to methanol or formic acid leads to accumulation of acid in the body. Formic acids inhibits cytochrome oxidase, causing decreased synthesis of ATP. This is followed by anaerobix glycoysis and lactic acidosis. At the same time and because of the acidosis, the generation of superoxide anions and hydroxyl free radicals is enhanced leading to membrane damage, lipid perioxidation and mitchondrial damage. Mitchondrial is the nucleus of the cell and how the cell will grow normally. This and the decreased pH in acidosis allows the influx of calcium into cells. Please make sure you read my column on Minerals, Essential Fatty Acids And The pH Factor. The mitochondrial dysfunction form formaldehyde buildup may be secondary to calcium overload in the mitochondrial, regardless, the final outcome is cell death. The untimely death of healthy cells. Due to a lack of a couple of key enzymes, humans are many times more sensitive to the toxic effects of methanol or formaldehyde than animals. Therefore, tests of Aspartame or methanol on animals will not accurately reflect the danger for humans.
As pointed out by Dr. Woodrow C. Monte, Director of the Food Science and Nutrition Laboratory at Arizona State University. Dr. Monte stated, "There are NO human or mammalian studies available to evaluate the possible mutagenic, tetraogenic or carciogenic effects of chronic or continued exposure or administration of methyl alcohol." However, aspartic acid, phenylalanine and methanol and its breakdown by-products have a cumulative effect due to rapid absorption and slow excretion in the body. These can be measured, however, no long term chronic ingestion studies of the "real world deadly effects" of Aspartame have been done on humans.
FORMIC ACID IS LISTED AS A PESTICIDE WITH THE EPA
http://www.epa.gov/pesticides/biopesticides/ingredients/factsheets/factsheet_214900.htm
Formic acid was registered (licensed for sale) as a pesticide active ingredient on January 28, 1999.
Are The Adverse Effects of Aspartame Dose Related?
The FDA claims that a daily dose of up to 50 mg/kg of Aspartame to body weight is safe. Estimated consumption of regular users is 2-10 mg/lg body weight. However, some people have suffered aspartame related disorders with doses as small as that which is in single stick of chewing gum.
In pregnancy, the effects of Aspartame can be passed directly to the fetus, even in very small doses. Even small doses of methanol in the blood stream can damage vision.
Folic Acid is believed by most researchers to play a large role in protecting cells and tissue from methanol poisoning by increasing the conversion of formic acid to carbon dioxide and water. (Roe, 1982, Tephyly 1984, DHHS 1993a). Persons who have a folic acid deficiency are most likely to be much more susceptible to damage from chronic methanol ingestion or aspartame ingestion. Nutritional differences among individuals such as folic acid deficiency may play an important part in the ability of an individual to metabolize formate, [methanol].
Methanol ingestion may even be more dangerous for persons taking certain pharmaceutical prescriptions as well. The drug Disulfiram, known as Antabuse, which is currently taken by over 400,000 persons in the U.S. inhibits the activity of aldehyde dehydrogenase in the liver. Animal experiments show a significant increase in toxicity of methanol because of this. Posner, (1975) lists research on several pharmaceutical drugs which shows drugs may present additional health hazards when taken with Aspartame because of the methanol exposure. These other drugs are sulfonylureas, ( for diabetics ), metronidazole, ( antibacterial ), and allopurionol (reduces uric acid ).
How and When Aspartame Was Discovered
In December, 1965, while creating a bioassay, James Schlatter was recrystallizing Aspartame from ethanol. The mixture spilled onto the outside of the flask and dried. Some of the powder got onto his fingers and when he licked his fingers, he noticed a strong sweet taste. He reported this finding in 1966 but did not mention the sweetness.
In 1969, investigators first reported the discovery of this new artificial sweetner, aspartame in the Journal of the American Chemical Society. Please note that this is a report published by Chemists. In the report, it was hailed as being 100-200 times sweeter that sucrose and without the unpleasant after taste.
In 1969, former FDA Commissioner, Dr. Herbet L. Ley said, "The thing that bugs me is that people think the Food and Drug Administration (FDA) is protecting them, it isn't. What the FDA is doing and what the public thinks it is doing is as different as night and day."
Now, keep the above quote in mind as you read the rest of the story.
The Study That Should Have Stopped The Approval Then, But Didn't In 1970, the discovery of Aspartame is officially reported in the well known publication, Science. In this same year, G.D. Searle Inc., approached Dr. Harry Waisman, Biochemist, Professor of Pediatrics, Director of the University of Mental Retardation Research and a respected expert in phenylalanine toxicity, to conduct a study of the effects of Aspartame on primates. The study was begun on January 15, 1970 and terminated around April 25, 1971, some 16 months later. Seven infant monkeys were given Aspartame with milk. One died after 300 days. Five others out of the seven had grad mal seizures. The actual results were hidden from the FDA when G.D. Searle Inc. submitted its' initial applications for the approval of Aspartame as a food additive. These results from this study were later read into the Congressional Record, 1985a, Gross 1976b, page 33 of the US Senate 1976b.
G.D. Searle, Inc. denied knowledge of or the involvement with the initiation, design or performance of negative studies of aspartame. Yet, false results in over 150 studies funded by G.D. Searle Inc. on aspartame and products, were submitted to the FDA bearing the Searle Pathology-Toxicology project number. Both Dr. Waisman and G.D. Searle were responsible for the study design of the studies. A number of false statements were made by G.D. Searle including that the animals were unavailable for purchase for autopsy after the termination of the study. There is more detail about this study on the research studies page. Also in 1970, neuro scientist and researcher Dr. John W. Olney found that oral intake glutamate and asparate, ( Aspartame) and cysteine, all excitotoxics amino acids cause brain damage in mice. One would think that with these studies this would have put at end to it being approved. No, it gets worse for consumers.
An internal G.D. Searle memo layed out the strategy for getting Aspartame approved by the FDA. The following is the exact wording of this internal memo. "At this meeting ( with FDA officials ), the basic philosophy of our approach to food and drugs should be to try to get them to say "Yes", to rank the things that we are going to ask for... so we are putting first those questions we would like to get a "Yes" to, even if we have to throw some in that have no significance to us, other than putting them in a "Yes" saying habit. We must create affirmative atmosphere in our dealing with them. It would help if we can get them or get their people involved to do us any such favors. This would also help bring them into subconscious spirit of participation."
During 1970, the FDA banned the sweetener cyclamate. Robert Scheuplein, acting Director of the FDA's Toxicological Services Center for Food Safety and Applied Nutrition was quoted as saying, "the decision to ban cyclamate was more a matter of politics than science." Keep that in mind for later.
In 1971, Ann Reynolds, a researcher who was hired by G.D. Searle Inc and who had done research for the Glutamate (MSG) Association, confirmed Aspartame's neurotoxicity in infant mice. This was after researcher, Dr. John W. Olney informed G.D. Searle that aspartic acid caused holes in the brains of mice. G.D. Searle did not inform the FDA of this study until AFTER Aspartame's approval by the FDA. None of these tests submitted by G.D. Searle to the FDA contradicted these findings, (Olney 1970, Gordon 1987, page 493 of the U.S. Senate 1987 hearings.) Yes, there were hearings later and what you'll learn later will be a shocker.
Early on, FDA Toxicologist, Dr. Adrian Gross discovered some irregularities in the submitted tests of the G.D. Searle drug, Flagyl. G.D. Searle did not respond for another two years. This is important to note because in 1987, Dr. Gross who is now at the EPA, finds severe irregularities in the monkey tests Searle had submitted in 1971, some fifthteen years later. You can read the details of this report/study the research page. On March 3, 1973, G.D. Searle petitioned the FDA for approval to market Aspartame as a sweetening agent. Here's a little background information. Back in August, 1970, G.D. Searle conducted two 78 week toxicity studies on rats for what was to become a best selling heart medication, Aldactone. One study conducted at G.D. Searle and one at Hazelton Laboratories. We need to back track a little time wise for important discovery of other prior findings. The following is a timeline of events starting in 1972.
In March 1972, the rats used in a study of aspartame were autopsied. The pathology slides were analyzed by an independent Board Certified Pathologist, Dr. Jacqueline Mauro. She discovered that Aspartame appeared to induce tumors in the liver, testes and thyroid of all of the rats. The report was submitted to G.D. Searle by Dr. Mauro. It was known as the MBR Report. Dr. Donald Willigan, employed at G.S. Searle did the other evaluation of the 1,000 slides. The Willigan Report was more to G.D. Searle's liking because it revealed significant increase in thyroid and testes tumors than liver tumors. The FDA was more concerned about liver tumors than the others. Needless to say, the Willigan report was submitted to the FDA and not Dr. Mauro's MBR Report. G.D. Searle did not disclose the MBR Report to the FDA until August 18, 1875, 27 months after Dr. Mauro gave the report to G.D. Searle. G.D. Searle claimed they did not submit Dr. Maruo's report because of a simple oversight. How convienent of an excuse.
In an FDA memorandum dated September 12, 1972, Martha M. Freeman, M.D. of the FDA Division of Metabolic and Endrocrine Drug Products addressed the adequacy of the information submitted by G.D. Searle in their petition to approve Aspartame. She stated, "Although it was stated that studies were also performed with diketopiperazine, (DKP), an impurity which results from the acid hydrolysis of Aspartame, no data are provided on this product." She further stated, "It is not feasible to extrapolate results of such single dose testing to the likely condition of use of Aspartame as an artificial sweetener." Dr. Freeman also noted, "Chemistry - no information is provided other than formula for Aspartame and its' diketo-piperazine. Pharmacology - Reference is made to 2 year rat studies, but no data are provided on acute or chronic toxicity."
It important to note here that Dr. Freeman confirmed the inadequency of single dose tests of Aspartame as early as 1973. Dr. Freeman also stated, "Clinical evidence of protocols nor curriculum vitae information are provided for the 10 completed clinical studies. Results are reported in narrative summary form. No information is given as to the identity of the reporting labs, methodology, or normal values. No pharmacokinetic data are provide on absorption, excretion, metabolism, half life; nor bio availability of capsule vs. food additive administration."
In Dr. Freeman's conclusion in the report, she said, "The administration of Aspartame as reported in these studies at high dose levels for prolonged periods constitutes clinical investigational use of a new drug substance, not a food additive. The information submitted for our review is inadequate to permit a scientific evaluation of clinical safety." Basically, Dr. Freeman is saying that G.D. Searle has provided no reliable long term use studies of Aspartame. in addition, since then, the NutraSweet Company has flooded the scientific community with single-dose studies. Single-dose means basically that the studies were long term ingestion of Aspartame over time. Here's a little more background about NutraSweet and G.D. Searle.
Ninety percent of the 113 Aspartame studies which Searle submitted to the FDA in the early 70's to mid 70's were all described by the FDA as "pivotal". Eighty percent of these studies/tests were conducted and paid for by G.D. Searle or by their major contractor Hazelton Laboratories. Dr. J. Richard Crout, acting director of the FDA Bureau of Drugs in 1974 stated, "The information submitted for our review was limited to narrative clinical summaries and tabulated mean values of laboratory studies. No protocols, manufacturing controls information or pre clinical data were provided. Such deficiencies in each area of required information precluded a scientific evaluation of the clinical safety of Aspartame."
In August, 1974, Dr. John Olney's study showed that Aspartame could very well cause brain tumors, especially in children. G.D. Searle's responses to queries about the testing of their drug Flagyl, an antiparasitic drug, and unexplained side effects from other drugs they developed and information from Dr. Olney started a controversy within the FDA as to the safety of Aspartame.
In July 1975, FDA Commissioner, Dr. Alexander Schmidt, appoints a special task force to look at 25 studies for the drugs, Flagyl, Aldactone, Norpace and the food additive, Aspartame. All made by G.D. Searle. This special task force was headed by Philip Brodsky, FDA's Lead Investigator and assisted by FDA toxicologist, Dr. Adrian Gross. The men were particularly interested in the "pivotal" studies. Dr. Schmidt stated, "if a study is found to be 'pivotal', then the test is not valid and must be repeated." During this same year, G.D. Searle Executives admit to payments to employees of certain foreign governments to obtain sales of their products. On July 10, 1975, Senator Edward Kennedy chaired a hearing on drug-related research before the Senate Sub-Committee. Preliminary reports of discrepancies discovered about G.S. Searle were discussed. The negative findings of the FDA Task Force were later presented at further hearings on January 20th, 1976. ( US Senate 1976a, and April 8, 1976, Senate 1976b).
On December 5, 1975, the FDA put a hold on the approval of Aspartame due to the preliminary findings of the FDA Task Force. Although it had already entered the market in some food products from July 1974 to December 1975. The Public Board of Inquiry is also put on hold. The evidence of the Aspartame pivotal studies were protected under FDA seal on December 3, 1975.
The following is a real shocker and joke in my opinion.
On January 7, 1976, G.D. Searle submitted to the FDA their proposal for the adoption of "Good Laboratory Practices". G.D. Searle's input was then used in the FDA's adoption of their own Good Laboratory Practices. Talk about buying the farm !
In March, 1976, the Special FDA Task Force that had been re-evaluating those "pivotal" studies of G.D. Searles Aspartame found these conclusions, these are the exact "quotes", the actual report is very long.
a) "At the heart of the FDA's regulatory process is its' ability to rely upon the integrity of the basic safety data submitted by sponsors of regulated products. Our investigation clearly demonstrates that, in the case of the G.D. Searle Company, we have not basis for such reliance now." b) "Some of our findings suggest an attitude of the disregard for FDA's mission of protection of the public health by selectively reporting the results of studies in the manner in which alley the concerns of the questions of a reviewer." c) "Excising masses, ( tumors) from live animals in some cases without histologic examination of the masses and in others not reporting these to the FDA." d) "Failure to report to the FDA all internal tumors present in the experimental rat studies." e) "G.D. Searle stored animal tissues in formaldehyde for so long that it destroyed the tissue." f) "Instead of performing autopsies on rhesus monkeys that suffered seizures after being fed Aspartame, the company financed a new money seizure study with a different methology that showed no problems." g) "Animals which had died were sometimes reported as alive and vice a versa. This included 20 instances of animals reported as dead and then reported as having vital signs in normal status again at subsequent observation periods." h) "In at least one study, the Aspartame 52 week monkey study, the protocol was written after the study was initiated." i) "Searle technical personnel failed to adhere to protocols, make accurate observations, sign and date records, and accurately administer the product under tests and proper lab procedures." j) "G.D. Searle delayed the reporting of alarming findings." k) "In the Aspartame 46 weeks hamster study, blood samples reported in the submission to the FDA as 26 week values were found by our investigators as being in fact, values for different animals which were bled at the 38th week. Many of these animals were dead at the 38th week."
There are many more negative findings that the Special Task Force found and listed. The ones listed above are the most serious. Basically, what all this means is this. The G.D. Searle studies were seriously flawed and what was provided in their studies, was manipulated to suit their own output of what they wanted the results to be. FDA Toxicologist and Task Force Member stated this in his conclusion in the report.
"They, G.D. Searle lied and they didn't submit the real nature of their observations because had they done that, it is more likely a great number of these studies would have been rejected. Searle took great pains to camouflage these shortcomings of the studies."
He also stated, "Searle evidently filtered and simply presented to the FDA what they wished the FDA to know. G.D. Searle did other terrible things. For instance, animals would develop tumors while they were under study. They would then remove these tumors from the animals without reporting them".
Senator Kennedy at a April 6, 1976 hearing before the Senate Sub-Committee stated after reading this report said, "The extensive nature of the almost unbelievable range of abuses discovered by the FDA on several major Searle products is profoundly disturbing." Need I say any more? In July 1976, the FDA finally decided to investigate 15 key Aspartame studies submitted by G.D. Searle in which the 1975 Special Task Force Study found serious problems. The problem here was G.D. Searle representatives met with the FDA and convinced them to allow G.D. Searle to "hire" a private agency, University Associated for Education in Pathology and pay them, $500,000 to "validate" 12 of the studies. In 1977, Donald Rumsfeld, ( presently our current Secretary of Defense in the Bush Adminstration), who was a former member of the U.S. Congress and the Chief of Staff in the Gerald Ford Administration was hired as G.D. Searle's President. Here again, Searle is paying the way into the political arena to make sure Aspartame gets approved using Rumsfeld's political connections. Rumsfeld was also on the Board of Directors of the Chicago Tribune which wrote "glowing" reports about the NutraSweet Company and the safety of Aspartame.
1977 - FDA Chief Counsel Recommends to U.S. Attorney to Charge G.D. Searle
On January 10, 1977, FDA Chief Counsel, Richard Merrill, recommends to the U.S. Attorney Sam Skinner, in a 33 page letter detailing the violations of the law and that a grand jury be set up to investigate G.D. Searle. In this letter is the following paragraph.
"We request that your office convene a Grand Jury investigation into apparent violations of the Federal, Food, Drug and Cosmetic Act, 21 U.S.C. 331(e), and the False Reports to the Government Act, 18 U.S.C., 101 by G.D. Searle and Company and three of its' responsible officers for their willful and knowing failure to make reports to the Food and Drug Administration required by the Act, 21 U.S.C. 355(i), and for concealing material facts and make false statements to establish the safety of the drug Aldactone and the food additive Aspartame."
Merrill went on to specify the most crucial reports. The 52 week toxicity study on infant monkeys and the 46 week toxicity report on hamsters. Many of the animals that G.D. Searle claimed had blood drawn from were actually dead at the 38th week. On January 26, 1977, G.D. Searle's law firms, Sidley & Austin requested a meeting with U.S. Attorney Samuel Skinner before a grand jury convened. One of the representatives at that meeting was Newton Minow, who was on the Board of Directors of the Chicago Tribune, ( remember, Rumsfeld is on the Board also ).
On March 8, 1977, in a confidential memo to aides, while U.S. Attorney Samuel Skinner is supposed to be pushing for fraud indictments against G.D. Seale, states in the memo that he has begun preliminary employment discussions with G.D. Searle's law firm, Sidley & Austin. Are you getting the drift of what is starting to occur?
On April 13, 1977, a U.S. Justice Department memo urged U.S. Attorney Skinner to proceed with Grand Jury investigations of G.D. Searle because the Statue of Limitations on prosecution would run out shortly, October 10th, 1977 for the monkey study and December 8, 1977 for the hamster study.
U.S. Attorney, Skinner then withdraws from the case. On July 1, 1977, he resigns and goes to work for G.D. Searle's law firm, Sidley & Austin. Thomas Sullivan is appointed Skinner's successor. Sullivan assigns the case to
Assistant U.S. Attorney, William Conlon who convened a Grand Jury, but he let the Statue of Limitations run out on the Aspartame charges. Fifthteen months later, Conlon accepted a job with the law firm representing G.D. Searle,
Sidley & Austin and was working along side his old boss, Samuel Skinner. Believe it, this is what happened!
Shortly after this, Robert McConnell who was the Director of G.D. Searle's Department of Pathology and Toxicology which oversaw most of the Aspartame research took a three year sabbatical. Mr. McConnell was named in Richard Merrill's letter to the U.S. Attorney Samuel Skinner. According to McConnell's attorney, his client was awarded a $15,000 bonus and asked to take a three year sabbatical for which he received $60,000 a year, a total of $180,000.00 The reason, he was a political liability in the wake of all this.
Philip Brodsky, the Lead Investigator for the original FDA Task Force looking into G.D. Searle's coverup of the studies retired. He stated his reason for retiring was the disclosure of the the 1975 Task Force findings before the U.S. Congress, Senator Kennedy's hearings in 1976, had become "politicized". As Gregory Gordon put it in his UPI Investigative article. Brodsky said in this article about the hearings, "The main witnesses, Searle executives and top FDA officials uninvolved in the investigation gave the "wrong" answers to the wrong questions...they didn't even let the experts answer the questions."
In August, 1977, the Bressler Report pertaining to the three key Aspartame studies, E6, E77/78 and E89 was released. These are some of the findings from the following persons and page numbers in the report. These reports were also given to members of the Senate Committee investigating this matter, some in 1989 and 1987. Mullarkey, 1994b, page 11, 48; Farber, 1989, page 110-112, Verrett, 1987, page 385 of the U.S. Senate Hearings.
In one study, 98 of the 196 animals died were not autopsied until as much as one year later. Because of the delay, much of the animal tissue could not be used and at least 20 animals had to be excluded from postmortem examinations. The original pathology sheets and pathology sheets submitted to the FDA showed differences for 30 animals. One animal was reported alive at week 88, dead from week 92 through 104, alive again at week 108, and finally dead at week 112. ( Talk about bringing the dead back to life. ) An outbreak of an infectious disease was not reported to the FDA Tissue from some animals were noted to unavailable for analysis on the pathology sheets, yet results from an analysis of this "unavailable" tissue was submitted to the FDA. There was evidence that the diet mix was not homogenous allowing the animals to eat around the test substance. This evidence included a photo and statements signed by a lab technician. This means they had no control on how much the test subjects were ingesting of Aspartame. Not a valid test. Fifthteen fetuses from animals in one experiment were missing. There was no protocol until one of the studies was well under way. Animals were not permanently tagged to prevent mixed up of data recording and autopsies Jerome Bessler in his report to the Senate said, "The question you have got to ask yourself is; because of the importance of this study, why wasn't greater care taken?" He further stated, " The study is highly questionable because of our findings. Why didn't Searle, which their scientists who supposedly closely evaluated this, knowing fully well that the whole society from the younger to the elderly, from the sick to the healthy...will have access to this product, ( Aspartame ). "
But wait...there is yet another report to be done. Another Special Task Force is formed to investigate the Bressler Report.
How many times does the FDA need to be told that the studies were flawed?
H.R. Roberts, Director of the FDA Bureau of Foods, created yet another five person task team to review the Bressler Report. This is not the same Dr. Richards that wrote the book, Aspartame Disease, An Ignored Epidemic. The review was done by a team at the Center for Food Safety and Applied Nutrition, ( CFSAN Report.) However, H.R. This Roberts, Director of hte FDA Bureau of Foods, would leave the FDA later on to become Vice President of the national Soft Drink Association who was looking at Aspartame as the next cheapest sweetener for soft drinks. FDA Toxicologist, Jacqueline Verrett was then appointed to Senior Scientist of the Bureau of Foods Task Force.
On September, 23, 1977, H.R. Roberts received the report from the Bureau of Foods Task Force. The report was a joke. They stated the following:
They had reviewed the Bressler Report and claimed that Searle had conducted all the stidues and that the FDA Bureau of Foods should accept the studies.
However, Senior Scientist of the FDA, Jacqueline Verrett did not agree with this report. She left the FDA when she openly discussed the Task Force with UPI Investigative reporter Gregory Gordon. Ms. Verrett said, "Members were barred from stating opinions about the research quality. It seemed pretty obvious that somewhere along the line they ( bureau officials ), were working up to a "whitewash". Verrett now a private consultant said she and other members wanted to come out and say they this whole experiment was a disaster and should be disregarded. In her testimony before the U.S. Senate, Verrett said, "We were looking at a lot of details and easy parameters in this study. When the foundation of the study was looked at, the diet and all of the other things were worthless. We were talking about the jockey when we should have been talking about the horse that had weak legs. The study was built on a foundation of sand. They told us in no uncertain terms that we were not to comment on the validity of the study. I think the studies should have been thrown at from day one and the GAO definitely did not objectively evaluate these studies, how could they, they had no one of experience to determine such." In March of 1979, the FDA somehow concluded that the G.D. Searle's Aspartame studies could be accepted.
This is what should be questioned.
How could the FDA come to this determination after all of the testimony before the U.S. Senate? The FDA however, next, convened the Public Board of Inquiry, (PBOI).
In April of 1979, the FDA outlined the specific questions which were to be addressed by the Public Board of Inquiry, (PBOI). The FDA limited the scope of the PBOI to the Federal Register 1981. The following questions were addressed again for the up tenth time concering aspartame.
1) Whether the ingestion of Aspartame either alone or together with glutamate, (MSG) poses a risk of contributing to mental retardation, brain damage, or undesirable effects on the neuroendrocrine regulatory systems. 2) Whether the ingestion of Aspartame may induce brain neoplasms ( tumors ) in the rat. 3) Based on answer to the above questions: a) Should Aspartame be allowed for use in foods, or instead should approval of Aspartame be allowed? b) If Aspartame is allowed for use in foods, i.e., if its' approval is not withdrawn, what conditions of use and labeling and label statements should be required, if any?
Dr. John Olney, researcher who had already performed numerous negative result studies, along with G.D. Searle and the FDA's Bureau of Foods were allowed to nominate scientists for the three person PBOI panel. It is important to note that the scope of the review was very limited despite all of the various adverse reactions that had already been reported to the FDA by consumers. The PBOI disallowed again, any discussion of the validity of the studies or experiments because it accepted the word of certain FDA officials, or were forced to accept that the studies and experiments had been "validated", which in fact they had NOT been. The PBOI Panel named three members and scientists. Dr. Olney objected strongly to Dr. Vernon Young on the grounds of conflict in interest and lack of qualifications in this area. Dr. Young had already written pro Aspartame articles in collaboration with G.D. Searle. Dr. Olney argued that the question of aspartic acid neurotoxicity should be looked at by a neuropathologist and not by Dr. Young who trained in Nutrition and Metabolism. Dr. Olney's objections were overruled by FDA Commissioner Sherwin Gardner at the time.
Dr. Walle Nauta one of the other three members of the PBIO Panel testified before Congress, Page S6493 of Congressional Record, 1985a). Dr. Nauta stated, "It was a shocking story we were told about Searle's animal testing, but, there was no way we could go after it. We had absolutely no way of knowing who was right. We had to take the FDA's word that the studies were valid. I would have definitely considered other tests and factors if I had known that Aspartame was planned for us in soft drinks." This can be found in ( Page SS5503 of Congressional Record, 1985a). This is when the soft drink coverup began and I'll get into that a little later on. In 1980, the Public Board of Inquiry voted unanimously to reject the use of Aspartame until additional studies on Aspartames potential to cause brain tumors could be performed. The PBOI was particularly concerned about experiment E33/34. This was an experiment where 320 rats received Aspartame. What was seen was a much higher percentage of animals in the Aspartame group developed tumors than in the control group. Also experiment E70 was of concern. Eighty rats had unusually high numbers of tumors. The problem they did not answer and this was due to Dr. Young's inexperience was this question: was it safe to add Aspartame to glutamic acids also known as MSG. MSG was already adultering the food supply. The safety plasma level that Dr. Young came up with was seriously flawed. It was the level at which half of the animals developed brain damage. These errors of Dr. Young threw the safety question of aspartic acid as part of Aspartame into doubt. This will be addressed later on in detail.
On January 21, 1981, the day after Ronald Reagan took office as President, G.D. Searle reapplied for the approval of Aspartame. G.D. Searle submitted several new studies along with their application. It was believed that Reagan would certainly replace Jere Goyan, the FDA Commissioner. G.D. Searle's President, Donald Rumsfeld, [ Rumsfeld is presently in 2006 - the U.S. Secretary of Defense ], connection to the Republican Party was probably why Searle re-applied like they did, one day after Reagan took office.
According to a former G.D. Searle salesperson, Patty Wood-Allott who testified before the U.S. Senate in 1987, said G.D. Searle President, Donald Rumsfeld, told his saleforce the following, "He would call in all his markers and that no matter what, he would see to it that Aspartame would be approved that year." And this man would someday be running the Department of Defense?
In March, 1981, a five member panel of scientists was established again. Here we go again folks, another review panel. The U.S. Government sure wastes a lot of tax money on these things. FDA Commissioner Jere Goyan appointed the panel to review the issues raised by the PBIO, ( Public Board of Inquiry ).
In April 1981, Arthur Hull Hayes Jr. was appointed to the FDA as Commissioner. On May 18, 1981, three of the scientists in the five member new review panel sent a letter to the panel lawyer discussing their concerns about Aspartame. Those three scientists were Satva Dubey, FDA Chief Statistical Branch, Douglas Park, Staff Science Advisor, and Robert Condon, Veterinary Medicine. Dubey thought that the brain tumor data was very worrisome and that he could not recommend approval of Aspartame. In another study, Dubey said that key data appeared to have been altered. Douglas Park said that the panel lawyer, Joseph Levitt hurried the panel to decide the issue. They wanted the results yesterday he said. Park said in Douglas Gordon's UPI report, "We really didn't have the time to do the in-depth review we wanted to do." Park said that Levitt met frequently with Hays, the FDA Commissioner, and was obviously getting pressure to get a resolution and a decision made, a favorable one.
Three of the five scientists on the commissioners review panel opposed the approval of aspartames. So...it was then decided to bring in a toxicologist for his opinion on isolated issues. His name was Barry Rosloff. Goyan said if the decision had been his, he would never had brought in the sixth person. While the panel did not vote, it ended in a 3-3 split. Levitt, because of this split, then took it upon himself to circulate an approval, recommendation. He backed off when Dubey, Park and Condon objected. Levitt explained to them that he as not directed to draft the approval memo, but did so as a tactical step to break the weeks long impasse of the panel by forcing each scientist to state his views. This pressure ploy worked.
On July 18, 1981 Arthur Hays Jr., FDA Commissioner got his wish. He approved Aspartame for use in dry foods. Now here is the corruption, the truth, the facts.
Hays overruled the PBOI and ignored the law, Section 409 (c) (3) of the Food and Drug and Cosmetic Act, (21 U.S.C. 348) . It states that a food additive should not be approved if tests are inconclusive, ( Federal Register 1981 ). In an article in Common Cause Magazine, Florence Graves states that two FDA officials said that Arthur Hull Hayes Jr. wanted to push Aspartame approval through in order to signal reforms of the Reagan Administration. The "real reasoning" behind the FDA Commissioners decision will be brought out later. First though, you need the rest of the story.
G.D. Searle Now Wants To Gets Approval For Aspartame In Soft Drinks On October 15, 1982, G.D. Searle petitioned the FDA for approval to use Aspartame in soft drinks and children's vitamins. Please pay special attention to children's vitamins. Could this be the cause of why so many children are being diagnosed with Attention Deficit Disorder? On October 1, 1982, an amendment was attached to the Orphan Drug Act which encounraged the development of drugs for rare diseases. However, it also modified the U.S. Patent law. The amendment extended the patent on only one product - Aspartame by five years, ten days and seventeen days. The amendment did not mention Aspartame or G.D. Searle by name and there was no debate or discussion on the amendment.
The amendment was proposed by Senator Howell Heflin. The bill was brought up for vote by Senator Robert Byrd. Senator Byrd as you know was the Senator that gave away the Panama Canal. The bill was pushed through by Representative Henry Waxman and Orrin Hatch. G.D. Searle asked Senator Heflin to sponsor the amendment. Heflin received $9,000.00 in campaign donations from G.D. Searle company executives and their wives.
In addition, Senator Byrd received a $1,000 campaign contribution from the G.D. Searle CEO, Donald Rumsfeld, who presently in 2001 is the U.S. Secretary of Defense. These contributions were made before the amendment was proposed. Representative Waxman received a $1,500.00 contribution from the soft drink political action committee prior to his re-election and $1,000 each from Daniel Searle, Wesley Dixon, ( Daniel's Searle's brother-in-law ), and William Searle. Senator Hatch numerous times blocked hearings looking into the safety of Aspartame. This was a total of $14,500.00 that was contributed by G.D. Searle to these politicians.
Campaign laws need to be changed to ban all contributions from corporations and or corporation executives and their relatives pertaining to any issue that any politician may be reviewing as in this case.
I want to stress that Apartame is not a natural substance. It is a CHEMICAL! The reason given by politicians for extending the Aspartame patent to Searle was to rectify the lost marketing time that Searle lost caused by the ridiculously run FDA investigations. However, had Searle performed the studies with any kind of competence, Aspartame could have been approved quickly like any other FDA approved food chemical additive. But that would not have happened if proper research had been conducted, the findings would have seen that aspartame is not for human consumption. The main point with all of this is that if the studies had been performed properly, without them being altered and manipulated to hide the true data, it is very unlikely that aspartame would have ever been approved for use, period!.
Between 1979 and 1982, four FDA officials who took part in the Aspartame approval process went through the FDA revolving door. They took jobs in the industries closely linked with the NutraSweet® issue. NutraSweet® is just another name for Aspartame. For example: Mike Taylor was an FDA lawyer who represented the FDA Bureau of Foods at the PBIO Hearing and was part of the team that prevented the quality and validity of G.D. Searle's studies from being considered properly. Sherwin Gardner was the Deputy FDA Commissioner in 1979. In July 1975, Gardner signed the initial approval for Aspartames use in dry foods. Here is a question. How could he give initial approval in 1975 when all the controversy about the studies was still up in the air?
In 1979, Sherwin Gardner became a Vice President of Grocery Manufacturers of America, Inc. Although Mr. Gardner claims he did not discuss Aspartame in his four meetings with the FDA within a year of leaving the agency or his twenty meetings with the FDA between 1980 and 1986. The organization he worked for dealt directly with the Aspartame products. How could he not talk about them? It is very unlikely that he would have been offered the job had he called for another delay in approval and had proposed that safety tests be conducted independently in order to the protect the public.
Stuart Pape was the Health and Human Services Chief Counsel for Foods Bureau from October 1976 to March 1979. He served as special assistant to the FDA Commissioner from March 1979 to December 1979. He participated in meetings and discussions on Aspartame as well as representing the FDA at the PBIO, ( Public Board of Inquiry ) Hearing. In December 1979, Mr. Pape was given a job by the law firm of Patton, Boggs and Blow. This law firm provided counsel to the National Soft Drink Association. Mr. Pape and Howard R. Roberts of the NSDA, Roberts formerly fought for approval of Aspartame at the FDA, met with the FDA twice in 1983 where Aspartame was discussed. In 1983, the NSDA suddenly and inexplicably withdrew their previously strongly worded objections of the use of Aspartame in diet beverages. Why? Was the "industry" using undue influence to get aspartame approved?
Aspartame Approved For Use In Soft Drinks
In early 1983, Acting FDA Commissioner, Mark Novitch who was the acting Commissioner because Arthur Hays Jr. was out of town on that day, approved the use of Aspartame in carbonated beverages and carbonated beverage syrup. What's wrong with this picture? Hayes gets a subordinate to approve the use. in addition, ignoring the FDA's own safety standards from the flawed Searle studies, they more than doubled the Acceptable Daily Intake of Aspartame from 20 mg/kg to 50 mg/kg. Now here is where it gets real disturbing and corrupted. Shortly after this approval, FDA Commissioner, Arthur Hayes Jr. resigns from the FDA under charges of improprieties and took a $1,000 a day position as the Dean of New York Medical College with G.D. Searle's public relations firm, Burston Marsteller. Are you getting the drift now of how Aspartame really got approved? On July 8, 1983, Dr. Woodrow Monte, Director of the Science and Nutrition Laboratory at Arizona State University files a petiton with the FDA objecting to the approval of Aspartame based on possible serious adverse effects from chronic intake of Aspartame. Dr. Monte was very concerned about the intake of methanol that would be be produced. Methanol is deadly to the immune system and the neurological systems of the human body. Dr. Monte also filed a petition with the Arizona Department of Health Services to ban Aspartame. In early 1984, the Arizona Department of Health Services completed studies showing that Aspartame in carbonated beverages breaks down into free methanol and other deadly toxins in 99 degree temperatures. The amount of methanol which broke down concerned the DHS enough that a ban on Aspartame was discussed for the state. ( Gordon 1987, page 507 of the US Senate 1987 ).
On February 17, 1984, the FDA denied Dr. Woodrow Monte and James Turner the opportunity to hold safety hearings on questions raised in their previous petition concerning Aspartame. Here again a political payoff blocked any further investigations into the safety of Aspartame.
Between August 23, 1984 and September 21, 1984, G.D. Searle officials contributed heavily to the campaign of Arizona House Majority Leader, Nurton Barr. The Committee to Relect Barr then gave campaign contributions to a number of state representatives. Don Aldridge, Karen Mills and Jan Bruer all received contributions eventually voted on the side of G.D. Searle in the Arizona Senate when the bill to ban Aspartame in Arizona was presented. This is another example of why campaign reform in this country must be done!
Dr. Richard Wurtman of MIT was quoted as saying that Dr. Gerald Gaull, a G.D. Searle Vice President came to his laboratory and threatened to veto his funding from the International Life Sciences Institute, (ILSI) after Wurtman quit his job as a G.D. research consultant and became a NutraSweet® opponent. Dr. Wurtman prior to this had conducted numerous studies showing the ill health effects of Aspartame. Can you tell me that politics did not play a role in getting Aspartame approved for use?
During 1984, 6,900,000 pounds of Aspartame was consumed in the U.S. alone.
Brain tumors start to increase in humans in 1985, leukemia cancers in both children and adults begin to increase.
In 1985, after suffering a $28 million dollar loss in the 1984 and selling off 30 subsidiaries after a suit filed by 780 women claimed G.D. Searle's intrauterine device caused them pelvic inflammatory disease, G.D. Searle sold out to the chemical company, MONSANTO. MONSANTO is the company that created the genetically altered canola seed. Read my column about canola oil. MONSANTO also created the genetically altered Starlink corn that kills monarch butterflies and causes allergic reactions in people. MONSANTO then created the NutraSweet® Company as a subsidiary company separate from G.D. Searle. Dr. Monte filed for reconsideration of his petition for a hearing in Arizona. He was granted a hearing scheduled for April 5, 1985. In that same month, in an unusual and secret maneuver, the Arizona Legislature removed the text in a Toxic Waste Bill and used it to pass a bill which banned the regulation of FDA approved food additives. This bill therefore more or less removed any hope of Dr. Monte ever having a hearing on the safety of Aspartame. This bill more or less in Arizona banned any regulation within the state of Arizona on any FDA approved food additive.
In 1985, 14,400,000 pounds of Aspartame was consumed in the U.S. alone.
On May 7th, 1985, the U.S. Senate heard testimony relating to an amendment put forth by Senator Howard Metzenbaum requiring the quantity of Aspartame to be labelled. It is nearly impossible for a person to determine what quantity of Aspartame they are ingesting unless it is labeled. Senator Orrin Hatch of Utah, again led the fight with G.D. Searle against the labelling amendment. The amendment was defeated for any quantity labeling of Aspartame in foods. Thus, the American public would never know how much they were ingesting of this deadly chemical called a food additive.
Those voting against the amendment were, Abdnor, Armstrong, Baucus, Bentsen, Biden, Bingaman, Boren, Boschwitz, Bradley, Bumpers, Cochran, Cohen, D'Amato, DAnforth, DeConcini, Denton, Dixon, Dole, Domenici, Durenberger, Evans, Ford, Garn, Goldwater, Gore, ( yes Vice President Gore ) Gorton, Gorton, Gramm, Gassley, Hatch, Hawkins, Hecht, Heflin, Heinz, Helms, Hollings, Humphrey, Inouye, Kassebaum, Kasten, Laxalt, Leahy, Levin, Lugar, Mattingly, McClure, McConnell, Mitchell, Murkowski, Nickles, Nunn, Packwood, Pressler, Pryor, Quayle, Reigle, Roth, Rudman, Sasser, Simpson, Stafford, Stevens, Symms, Thurmond, Tribe, Wallop, Warner, Wilson, Zorinky. You can thank each of these Senators for unleashing a deadly toxic chemical onto the American public. Some of these Senators are still in Congress and I strongly suggest that you contact each and everyone of them that are still in office and tell them you will not be voting for them in the next election.
Those voting for the above amendment were, Burdick, Byrd, Chafee, Chiles, Cranston, Dodd, Eagleton, Glenn, Harkin, Hart, Hatfield, Johnston, Kennedy, Kerry, Lautenberg, Long, Mathias, Matsunaga, Melcher, Metzenbaum, Moynihan, Pell, Proxmire, Rockefeller, Sarbanes, Simon, Spector. I strongly suggest that these Senators be contacted by you, my readers, demanding Senate public hearings on the safety of Aspartame.
On August 1st, 1985, Senator Metzenbaum tried again, introducing a bill entitled, "Aspartame Safety Act of 1985". It would have required the quantity labelling of Aspartame on food items and mandated that there me a moratorium on any new uses of Aspartame until independent studies could be conducted under the auspices of the National Institutes of Health. Testimony was submitted for the record. The bill was submitted to a Senate Committee where it died. So much for our politicians caring about the health of their constituents.
1986 - The Heat Gets Turned Up Against The FDA
The Community Nutrition Institute, (CNI), filed suit against the FDA in District Court claiming the FDA did not follow proper procedure in approving Aspartame for beverages and that they should have held a public hearing before giving final approval. What is very disturbing here, both the District Court and the Appeals Court dismissed the claim. The Appeals Court in Washington D.C. stated that the CNI, "failed to raise any material issues of fact that require the FDA to grant a hearing." The CNI issued a press release shortly afterwards, "Where the holding of a public hearing is no longer a responsible part of the food additive process, the FDA and the appeals court have increased the likelihood that unsafe food additives will reach the food market."
In July 1986, the U.S. General Acounting Office, (GAO), published the results of an investigation of five former government employees involved in the Aspartame approval process. They found five former government workers had taken high paying jobs in the Aspartame industry shortly after Aspartames approval. While these former employees actions are not illegal, it is a good example of how the U.S. Government and especially the FDA's "revolving door" helps certain powerful companies have near complete control over governmental actions.
This is why action must be taken to change the way the FDA conducts it approval process. Laws must be put in place that prohibits any FDA employee for going to work for any company for at least three years after any review has been done on any product. All research studies must be done by independent researchers, paid with taxpayer dollars and not corporate funds. That way, the studies will be free from any outside influence. Public hearings must be held on any approvals.
In 1986, 15,700,000 pounds of Aspartame was consumed in the U.S.
An abnormal unexplained increase in auto-immune diseases are suddenly starting to be seen across the U.S.
On October 12, 1987, the United Press reported that more than ten federal officials involved in the NutraSweet® Aspartame FDA decision took high paying jobs in the private sector linked to the Aspartame Industry.
On November 3, 1987 a hearing was held in a U.S. Senate Committe to address the issue of Aspartame safety and labelling. Senator Orrin Hatch successfully blocked any labeling requirements.
In June 1987, the U.S. General Accounting Office, (GAO) published the results of an investigation which looked at whether the FDA followed its' required approval process. The report concluded the following: "Because the FDA followed its required approval process in approving aspartame and monitors adverse reactions and ongoing aspartame research, the GAO is making no recommendations." What is very important here though is that the author of the above statement in the report, also specifically stated on the first page of his report, "We did not evaluate the scientific issues raised concerning the studies used for Aspartames approval or FDA's resolution of these issues, nor did we determine Aspartames safety. WE DO NOT HAVE SUCH SCIENTIFIC EXPERTISE."
Another words, this was a waste of taxpayers money, however, on the overhand, they did have the power to review the following issues for legal reasons, however they failed to even do these:
The GAO made numerous factual errors in their report. The FDA's own Investigator and Toxicologost Dr. Adrian Gross concluded in a statement after the GAO report was made public:
"Although in their report, the GAO expresses the view that the FDA 'followed its required process in approving Aspartame for marketing', I would sharply disagree with such an evaluation. Although the FDA may have gone through the motions or it may have given the appearance of such a process being in place, the people of this country expect and require a great deal more from that agency charged with protecting their public health in addition to mere facade or window dressing on the part of the FDA. They require a thorough and scientifically based evaluation by the Agency on the safety of the products it regulates. Unfortunately, this has clearly not been the case with the approval of Aspartame. And without this kind of assurance, any such process is nothing more than a farce and a mockery of what is truly required."
There, you have it in the words of one of the FDA's chief investigators that fought this issue. In other words, the studies that G.D. Searle provided to supposedly show the safety of Aspartame were nothing more than a farce. During 1987, 17,100,000 pounds of Aspartame are consumed in the U.S. Alone.
In 1987, NutraSweet® stopped providing consumption data to the USDA. It is not required by law. It should be in my opinion. It is much easier for NutraSweet® scientists to create inaccurate Aspartame consumption figures when the total number of pounds sold is not publicly available, or inaccurate when it is given out publicly. My opinion is that they did not want the total number of pounds disclosed because this could be a key indicator to tie in with the huge percentage increases in such diseases as MS, Lupus, auto-immune disorders, brain tumors. The FDA needs this information to correlate to disease states. And keep in mind...all of these diseases have dramatically risen since the introduction of Aspartame into the food supply. In August, 1988, Aspartame was approved for use in Brazil. MONSANTO used a massive advertising campaign and by the end of 1990, 150 products in Brazil were sweetened exclusively with Aspartame.
In May, 1990, NutraSweet®, ( MONSANTO ), opened a production facility in Sao Hose dos Campo, Brazil. There were no diet foods in Brazil in the 1980's. NutraSweet® efforts build a diet segment in Brazil and years later, significant increases in brain tumors, auto-immune diseases, children attention disorders are seen. NutraSweet® has begun to dose the population with their slow poision.
NutraSweet® ( MONSANTO ) then joined with its long time partner, Ajinomoto Company Inc. of Japan to begin the building of an Aspartame manufacturing plant in Gravelines, France. During this same time, NutraSweet® began a project to develop a new sweetener called, Sweetener 2000. It was said to be approximately 10,000 times sweeter than sugar. The chemical composition of this new sweetener was not detailed in MONSANTO'S Annual Report. NutraSweet® was to get this new sweetener ready for market by the end of the decade. In 1992, NutraSweet® signed agreements with the Coca-Cola CO. and Pepsico Inc., stating that "The NutraSweet® Company is their preferred supplier of Aspartame." NutraSweet® in one of their marketing statements stated that one of their options for increased sales in carbonated soft drinks is to prepare, "HIGH CONCENTRATION FORMULAS USING MORE Aspartame". This means more of these deadly toxic chemical in soft drinks. The FDA approved the NutraSweet® company's application to market Aspartame in bulk form to the bottlers. The patent for Aspartame expired on December 14, 1992, thus opening the market up now to other companies such a Holland Sweetener Company.
In mid-1993, NutraSweet® and long time partner, Ajinomoto Co. of Japan began producing Aspartame from their new production plant in Gravelines, France. NutraSweet® also begins a joint venture with Nestle Mexico to bring Aspartame into Mexico and to explore other uses of Aspartame in Mexico. Yes, it is Nestle, the company that makes those wonderful candy bars. In 1994, NutraSweet® introduces those little blue packages for tabletop use products in Mexico, Hungary, Uganda, Ecuador, Romania, Uruguay and Paraguay. They are intent on spreading this "plague" to all countries using this chemical poison. Aspartames net sales outside of the U.S. accounts for ten percent of all net sales for the companies.
Keep in mind that during the early 1980's and again in 1994, scientists at the National Institutes of Environmental Health Sciences, (NIEHS), proposed at least four times that the government's leading program for toxicology research fund new studies on the safety of Aspartame. After each of these requests, NIEGS officials decided not to pursue the research because officials at the FDA said they were satisfied with industry sponsored research that found no health risks. This is a down and out blatant lie by the FDA. No health risks ?
In 1995, Nutrasweet® announced plans to market Aspartame tabletop packets throughout Southeast Asia. They also plan to introduce it to India, and test market it in China. In a June 13, 1995 article which appeared in Food Chemical News, Thomas Wilcox, the FDA Edpidemlogy Branch Chief was quoted as saying, "FDA has no further plans to continue to collect adverse reactions reports or monitor research periodically done on Aspartame." Another words, they are going to ignore the more than 10,000 adverse reaction reports already filed with them and simply ignore any further research no matter how damaging it is against Aspartame. NutraSweet® is now marketing as Equal® in Shanghi, China, pushing this poison on 60 million innocent Chinese in the coastal cities.
In 1996, distinguished neuroscientist and researcher, Dr. John T. Olney publishes research showing that Aspartame is a brain tumor agent. He shows that Aspartame caused brain cancer in pre approval research. He shows a breakdown product of Aspartame causes mutations in vitro and that from 4 to 13 years after the approval of Aspartame, there has been a significant increase in the conversion of less deadly brain tumors in mice and more deadly brain tumors as seen in the pre approval monkey research. The MONSANTO Corp. and the FDA responded with irrelevant statements regarding the overall brain tumor rate.
March 27, 2000 Monsanto Company signed a definitive agreement to sell its sweetener ingredient business, including the NutraSweet® brand, to J.W. Childs Equity Partners II, L.P., a Boston based private equity firm with substantial experience in the food, beverage and food ingredient industries for $440 million dollars. The principal locations of the NutraSweet® business are in the Chicago, IL metropolitan area and Augusta, GA. MONSANTO also signed a definitive agreement to sell for $67 million in cash its equity interests in two European joint venture companies, NutraSweet® A.G., and Euro-Aspartame S.A., to Ajinomoto Co., Inc., Japan, Monsanto's long standing partner in the joint ventures. Monsanto is a wholly owned subsidiary of the Pharmacia Corp. J.W. Childs Associates L.P. has equity interests in General Nutrition Centers, Ghirardelli Chocolate Co., Empire Kosher Poultry and Chevys Fresh Mex Mexican restaurant chain. I ask my readers to avoid doing business with these companies or purchasing their products.
NutraSweet used to be the market leader in the $1.1 billion global sweetener market and is used daily by an estimated 350 million people in more than 100 countries. Splenda however, has taken the lead. Hello, is there anyone listening? That is 350 million people that are consuming a deadly toxic chemical daily.
What can you do to take this deadly toxic chemical out of our food supply? Many Things! I am calling upon on all my readers, in the U.S. and worldwide to get involved and stop this worldwide plague. Below are ways you can help spread the word and contact your Congressmen
WHO IS ASPARTAME.ORG?
The website aspartame.org is nothing more than a front for the aspartame industry promoting aspartame's so called safety. The website is owned by the Calorie Central Coucil. Here are the other details you will find very disturbing:
Calorie Central Council They are a client of the Kellen Company * In 1992, FDA appointed both Andrew G. Ebert, Ph.D., IGTC chairman, and Kristin McNutt, Ph.D. ,paid spokesperson for the IGTC, to the FDA Food Advisory Committee. As a food-industry pharmacologist and
toxicologist, Ebert has provided scientific and technical expertise for programs of many associations managed by The Kellen Company. Ebert continues, however, as an officer of the Robert H. Kellen Company.
IMMEDIATE WARNING!
J.W. Childs Equity Partners II, L.P., a Boston-based private equity firm which now owns NutraSweet® has applied to the FDA for the approval of Neotame in the United States. This must be stopped! The company states that it is 40 times sweeter than Aspartame. However, there have been NO INDEPENDENT FINANCED research studies on the safety of this new sweetener. Notice tame at the end. It basically is Aspartame. It simply has been genetically altered again. Has this made it more deadly? Please write your Congressman to keep this deadly chemical toxin from being approved for use in foods!
What You Can Do To Help Spread The Word About Aspartame! Write your Senators and Representatives. You can use this already prepared letter. Simply copy and paste into emails or letterheads. You can find a listing of your Senators and Representatives, which list email addresses, phone numbers, etc. on my site here. You can use this, form letter to write your Senators and Representatives. All you will have to do is copy and paste it into your email or your letterhead to mail. Also on this form, you can send us a copy of the email you have sent your Congressman. You will need to input your name, address, telephone number, email address. Then go to your email folder and copy your email into the text box area provided. This will send a copy of your letter to us. It is crucial that we know how many are writing their Congressman and to keep a master log copy of all letters sent to Congressman and or Senators. As you know, "letters" seem to get lost in Washington. This way, we can followup with these Senators to see if they have responded to you and what they are going to do about the issues addressed in the letter you have sent them if they do not respond to you.
Tell your family, friends about this column. Email them, phone them, whatever it takes. You can use this form letter to print out and to give to friends or copy and paste into emails or letterheads to family and friends. Print this letter out and give to your next door neighbor and ask him or her to copy it and pass it down the block. If we get this going, eventually everyone will know about these deadly additives in our food products!
* The Kellen Company
http://www.kellencompany.com
The Kellen Company is an employee-owned professional services company providing a full range of management, consulting and specialty services to associations and businesses since its founding in 1964. 160 employees operate from modern headquarters facilities in Atlanta, Brussels, New York,Tucson and Washington, D.C.
*Interesting the National Soft Drink Association is no longer listed as a client.
However... in 2001, they were representing them at the CODEX ALIMENTARIUS COMMISSION,
in Canada.
Kellen was co-authors of this paper in 2004:
Position
of the American Dietetic Association:
Use of Nutritive and Nonnutritive
Sweeteners
* they talk about aspartame
PDF COPY:
http://www.d-et.com/articlePool/sweetener.pdf
Robert Gilardi - CEO of KELLEN - testified at OSHA for the American
Compressed Association
http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=PREAMBLES&p_id=840
*Trying to discredit complaints of poor equipment from workers among Gas Suppliers...
Andrew G. Ebert is an Officer of the Kellen Company, since the company is employee owned, he owns stock in the company as well.
His nomination to the FDA Food Advisory Committee did not refer to his affiliation with the IGTC,
but listed him only as Senior Vice President of The Kellen Company. Ebert is also an active member of the Institute of Food Technologists(IFT). Daryl Altman, M.D., a spokesperson for the glutamate industry, worked for former IFT president
Al Clausi, Vice Chairman of Allerx, Inc. and itsmedical affiliate, The Food Allergy Center.
Altman has spoken publicly about the safety of monosodium glutamate, often with Taylor. The IFIC promotes them as speakers without mention
of the fact that they represent the glutamate industry. L.T. Chiaramonte, M.D., who has co-authored work for the IGTC with Altman, has served on the medical advisory board of The Food AllergyCenter.
Ebert continues to play a
major role for the IGTC. However, following exposure of the fact that research protocols used by IGTC sponsored researchers were flawed to the point of being fraudulent, including, for example, use of MSG and other reactive materials in
placebos provided to researchers by the IGTC, Ebert was replaced as IGTC chairman.
* Talk about an unethical conflict of interest and how could he have ever been appointed to an
FDA Board?
CLIENT LIST OF THE CC:
http://www.kellencompany.com/solutions_practice.html
Also in EUROPE:
http://www.kellencompany.com/about_europe.html
A Google search on the name "meaganUGA" shows that she has
posted on a few other sites about this same issue, each time not disclosing her
relationship to this council.
On GOOGLE for
MEAGANUGA
http://www.google.com/webhp?hl=en#hl=en&expIds=17259,17291,22881,24279,25981,26714,27006&sugexp=ldymls&xhr=t&q=MEAGANUGA&cp=9&pf=p&sclient=psy&site=webhp&source=hp&aq=f&aqi=&aql=&oq=&gs_rfai=&pbx=1&fp=c6affe93747c32d0
BOTTOM LINE: Do not believe anything that the Calorie Cental Council puts out about the so called "safety" of aspartame. They are paid to do that!
WHO OWNS ASPARTAME.ORG
Registrant:
ASPARTAME.ORG
Calorie Central Council
5775 G Peachtree Dunwoody Road NE
Atlanta, GA 30342
US
Domain Name:
ASPARTAME.ORG
Record expires on 25-Aug-2012
Record
created on 24-Aug-1996
Database last updated on
07-Mar-2006
One IMPORTANT thing we must do...
|
|
Important Information For My Readers In Australia
To my readers in Australia. On 31 July 2001 the Australia New Zealand Food Standards Council allowed a variation to the list of approved artificial sweetening substances to include a new substance Neotame. This is just a new variation of Aspartame, but is even more deadly. You must contact your government officials in Australia to ban both Aspartame and this new version Neotame.
This Books Provides The Scientific Evidence
Dr. H. J. Roberts, MD, FACP, FCCP, is a Board certified internist, internationally known medical consultant and independent researcher. He is on the Staff of Good Samaritan Hospital and St. Mary's Hospital in West Palm Beach, Florida. Dr. Roberts has written the book, "Aspartame Disease, An Ignored Epidemic", ISBN # 1-884243-17-7. I'm calling upon my network of Mothers nationwide. Print this entire column out along with the research pages. Take copies of it to your local school board hearings, your local PTA meetings and get the word out. Demand that school board officials BAN all foods containing Aspartame, MSG, and most important - foods containing hydrogenated oils. Print my column, "Hydrogenated Oils-Silent Killers" as well and take it with you. This means NO SNACK FOOD machines containing snacks with products containing hydrogenated oils. This also means NO soft drink machines, especially those containing any soft drinks sweetened with Aspartame.
Click on Research Studies to read the list of 90 other research studies showing the toxicity of Aspartame The study G.D.Searle did not want anyone to see showing the toxicity of Aspartame.
If you have enjoyed this column and have not signed up for the automatic notification of my column via email newsletter, and would like to receive such notification, CLICK HERE. You'll be taken to a form to request to be placed on the mailing list. And rest assured...your email address it not sold to outside third parties. Be sure to read "Hydrogenated Oils-Silent Killers", you will know how these deadly oils are causing many diseases, coronary heart disease, diabetes type II and a host of other health problems, even an increase risk of breast cancer in women. And be sure to read my column on the importance of minerals ~ ~ David Lawrence Dewey
 It is a must read. You can order it from Sunshine Sentinel Press, Inc., P.O. Box 17799, West Palm Beach, FL. 33416, TOLL-FREE number 800-814-9800. There website is, www.sunsentpress.com It contains 1,038 pages, nine appendices, bibliography and well worth the price of $75.00. You can visit Dr. Roberts website at, www.Aspartameispoison.com.
It is a must read. You can order it from Sunshine Sentinel Press, Inc., P.O. Box 17799, West Palm Beach, FL. 33416, TOLL-FREE number 800-814-9800. There website is, www.sunsentpress.com It contains 1,038 pages, nine appendices, bibliography and well worth the price of $75.00. You can visit Dr. Roberts website at, www.Aspartameispoison.com.
90 other Research Studies.
The study Searle did not want anyone to see.
|
IMPORTANT UPDATE: Make sure you read about the new documentary  Read About the Film - Click Here Do you want to die young with a diseased heart? Develop needless high blood presure? Develop diabetes type II ? If not - then you need to watch this new documentary ! THE FILM HAS WON FIVE FILM AWARDS ! |
Would you like to save up to 25% on your gasoline cost in your car or truck?
 Then read what actual users of this phenomenal device are raving about ! CLICK HERE |
Improve your health with these amazing water wands !
The water wand introduces passive natural energy waves into clean drinking water, fruit, vegetable and vitamin drinks. This process causes water molecules to shed excess minerals and other substances, which break down into finer more usable nutrients. Since the water molecule becomes lighter, you can drink more liquids. This process balances pH, transports nutrients, and absorbs more waste in the body at a faster rate. Drinking more water and fluids helps increase your rate of hydration, assimilation of nutrients and elimination (detoxification). This subtle energy is discernable and gives a feeling of well-being. red blood cells before and after use. CLICK HERE |
Do you care about your health, the health of your children, your family?
Then make sure you read my column:
Hydrogenated Oils - Silent Killers
Learn the truth about these deadly oils in our food supplies
Read about Greta Ferebee's and my efforts in a nationwide petition campaign to get these and other toxins out of the food supply. VISIT our website:

| © All Rights Reserved. Use of these articles is for personal use only. Any other use is strictly prohibited. Newspapers, syndicates or publications wishing to print his columns, email your request with details to Mr. Dewey's agent. Email Contacts for DL Dewey. For any other use, DLDEWEY for permission to use column or columns, detailing your request to use which column or columns and for what purpose. |
| HOME | Previous Columns | Email Contacts | Advertising |
|
©1996-2013 Rocky Mountain Publicity Last Modified: June 1, 2013 |